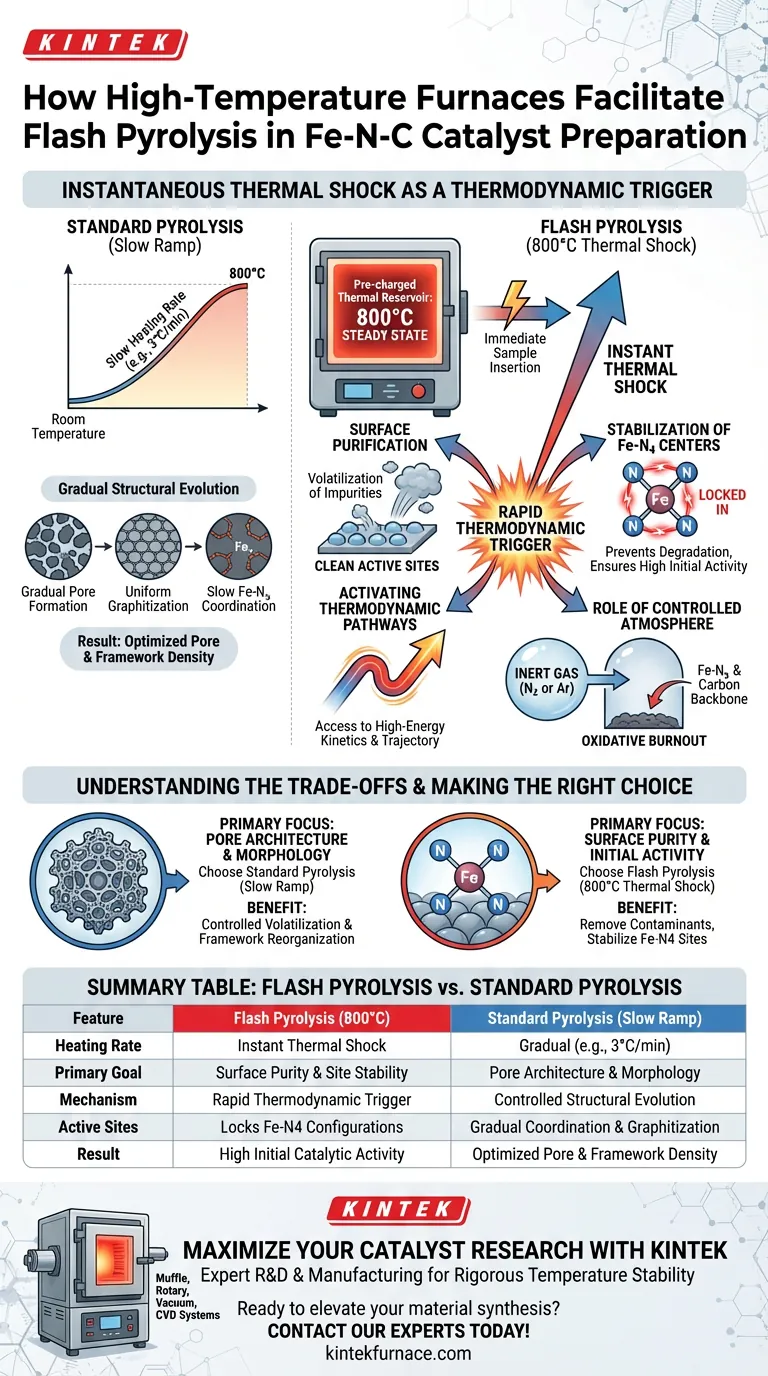
A high-temperature furnace facilitates flash pyrolysis by functioning as a pre-charged thermal reservoir. By maintaining a steady state of 800°C prior to sample insertion, it delivers an immediate thermal shock rather than a gradual temperature ramp. This instantaneous energy transfer is the critical driver for stabilizing the specific atomic configurations required for high-performance Fe-N-C catalysts.
Core Takeaway Unlike standard pyrolysis which relies on gradual heating, flash pyrolysis leverages thermal shock to instantaneously activate specific thermodynamic pathways. This rapid process is essential for removing surface impurities and locking in the atomic structure of Fe-N4 active centers, directly enhancing the catalyst's initial activity.
The Mechanism of Flash Pyrolysis
The Principle of Thermal Shock
The defining characteristic of this method is the rapid introduction of the sample into a furnace already preheated to 800°C.
Standard pyrolysis typically utilizes slow heating rates (e.g., 3°C/min) to gradually evolve the material. In contrast, flash pyrolysis subjects the precursor to an immediate, intense temperature jump. This shock is necessary to bypass intermediate heating stages and access high-energy reaction kinetics immediately.
Activating Thermodynamic Pathways
The instant exposure to 800°C triggers specific thermodynamic pathways that are not accessible during slow-ramp heating.
The rapid influx of thermal energy forces the material to undergo instantaneous chemical transformations. This unique thermal history directs the atomic organization along a specific trajectory that favors high-activity catalytic structures.
Stabilization of Fe-N4 Centers
The primary goal of this thermal shock is the stabilization of the atomic structure, specifically the Fe-N4 active centers.
Fe-N4 sites are the critical components responsible for catalytic performance. The flash pyrolysis method ensures these sites are formed and locked into a stable configuration, preventing them from degrading or aggregating which might occur during prolonged, slower heating cycles.
Surface Purification
Flash pyrolysis acts as a rapid purification step.
The sudden high temperature effectively strips away surface impurities. By instantly volatilizing unwanted byproducts, the process exposes the active sites, ensuring the material possesses high initial catalytic activity.
The Role of Controlled Atmosphere
Inert Gas Protection
While the thermal shock is the primary driver, the furnace must still provide a controlled atmosphere, typically using nitrogen or argon.
As noted in standard pyrolysis protocols, an inert environment is crucial to prevent the oxidative burnout of the carbon support. Even during the rapid "flash" step, the protection of the carbon backbone and the coordination of Nitrogen and Iron atoms (Fe-Nx) rely on the absence of oxygen.
Expulsion of Volatiles
The high-temperature environment facilitates the rapid expulsion of volatile decomposition products.
In standard methods, components like zinc or urea volatilize slowly to create pores. In flash pyrolysis, this expulsion is immediate, which contributes to the rapid "cleaning" of the catalyst surface mentioned above.
Understanding the Trade-offs
While flash pyrolysis offers distinct advantages, it represents a specific strategic choice in catalyst synthesis compared to standard methods.
1. Activity vs. Morphology Control Flash pyrolysis excels at enhancing initial activity and cleaning the surface. However, standard pyrolysis (slow ramping) allows for more precise control over the bulk morphological evolution, such as the gradual collapse of precursor frameworks (like ZIF-8) and the methodical formation of pore structures.
2. Thermal Shock vs. Uniformity The thermal shock method is aggressive. While it stabilizes Fe-N4 centers effectively, it subjects the material to high stress. Standard methods that heat from room temperature provide a gentler environment for the uniform graphitization of the carbon skeleton and the slow coordination of metal-nitrogen bonds.
Making the Right Choice for Your Goal
The decision to utilize a high-temperature furnace for flash pyrolysis versus standard pyrolysis depends on the specific deficiency you are trying to address in your material.
- If your primary focus is Surface Purity and Activity: Utilize flash pyrolysis at 800°C. The thermal shock will remove surface contaminants and stabilize the Fe-N4 active sites for maximum initial performance.
- If your primary focus is Pore Architecture: Consider standard pyrolysis with slow heating rates (e.g., 3°C/min). This allows for the controlled volatilization of pore-forming agents (like Zinc) and the organized reorganization of the carbon framework.
Flash pyrolysis is not merely a heating step; it is a thermodynamic trigger that prioritizes surface cleanliness and active site stability over gradual structural evolution.
Summary Table:
| Feature | Flash Pyrolysis (800°C) | Standard Pyrolysis (Slow Ramp) |
|---|---|---|
| Heating Rate | Instant Thermal Shock | Gradual (e.g., 3°C/min) |
| Primary Goal | Surface Purity & Site Stability | Pore Architecture & Morphology |
| Mechanism | Rapid Thermodynamic Trigger | Controlled Structural Evolution |
| Active Sites | Locks Fe-N4 Configurations | Gradual Coordination & Graphitization |
| Result | High Initial Catalytic Activity | Optimized Pore & Framework Density |
Maximize Your Catalyst Research with KINTEK
Precise thermal shock requires a reliable, pre-charged thermal reservoir. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to maintain the rigorous temperature stability needed for flash pyrolysis and Fe-N-C synthesis. Whether you need to lock in Fe-N4 active centers or meticulously control pore architecture, our customizable lab furnaces provide the exact thermodynamic environment your research demands.
Ready to elevate your material synthesis? Contact our experts today to find the perfect high-temperature solution for your lab!
Visual Guide
References
- Davide Menga, Michele Piana. On the Stability of an Atomically‐Dispersed Fe−N−C ORR Catalyst: An <i>In Situ</i> XAS Study in a PEMFC. DOI: 10.1002/celc.202400228
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What are the considerations for air atmosphere and cooling in Inconel 625 heat treatment? Optimize 3D Part Stability
- What critical function does a high-temperature atmosphere sintering furnace serve? Engineer Advanced Nuclear Fuels
- What is the difference between a vacuum furnace and an atmospheric furnace? Choosing the Right Thermal Process
- What is the purpose of introducing a nitrogen protective atmosphere during the continuous annealing of silicon steel?
- What is the function of a thermal oxidation furnace in MEMS growth? Create High-Quality Passivation Layers
- Why is a controlled atmosphere furnace required for 316L debinding? Ensure Structural Integrity & Zero Cracks
- What core role does a Pyrolysis Furnace play in aerospace-grade prepreg waste recycling? Achieve High-Value Recovery
- Why is a high-precision annealing furnace necessary for optical fibers? Control Nanoparticle Growth for Peak Performance



















