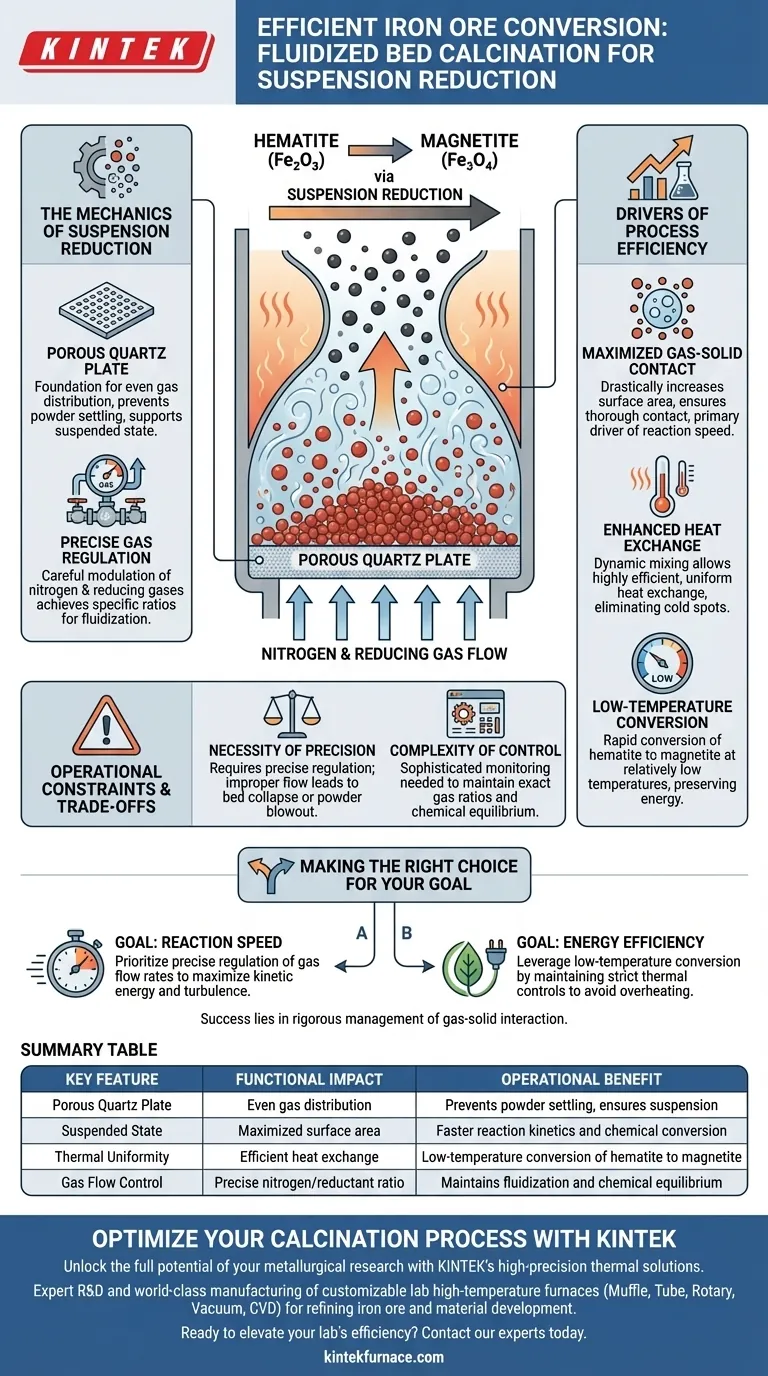
A fluidized bed calcination system maximizes efficiency by actively suspending iron ore powder in a gas stream above a porous quartz plate. By strictly controlling the flow rates of nitrogen and reducing gases, the system creates an optimal environment where solid particles behave like a fluid. This state facilitates the rapid chemical conversion of hematite to magnetite, even at relatively low temperatures.
The system's primary value is its ability to ensure thorough gas-solid contact through precise fluidization. This maximizes heat exchange and reaction kinetics, allowing for faster reduction without the energy costs associated with higher-temperature processes.

The Mechanics of Suspension Reduction
The Role of the Porous Plate
The foundation of the system is a porous quartz plate. This component serves as the distribution point for the gas stream.
It supports the iron ore powder while allowing gases to permeate upward evenly. This ensures the powder does not settle but remains in a dynamic, suspended state.
Precise Gas Regulation
Success depends on the careful modulation of nitrogen and reducing gases.
Operators must regulate the flow rates to achieve specific ratios. This balance is critical to maintaining the "fluidized" state and providing the necessary chemical reactants for the reduction process.
Drivers of Process Efficiency
Maximizing Gas-Solid Contact
The suspension of the powder drastically increases the surface area exposed to the reducing gases.
Unlike static beds where gas might channel through cracks, fluidization ensures thorough contact between the gas and every particle of ore. This contact is the primary driver of the reaction speed.
Enhanced Heat Exchange
Fluidized systems are renowned for their thermal uniformity.
The dynamic mixing of the suspended powder allows for highly efficient heat exchange throughout the chamber. This eliminates cold spots and ensures that the activation energy required for reduction is distributed evenly.
Low-Temperature Conversion
Because the contact and heat transfer are so efficient, the chemical transformation does not require excessive heat.
The system allows for the rapid conversion of hematite to magnetite at relatively low temperatures. This preserves energy while achieving the desired metallurgical phase change.
Operational Constraints and Trade-offs
The Necessity of Precision
The efficiency of a fluidized bed is entirely dependent on maintaining the "optimal state."
This requires precise regulation of gas flows. If the flow rate drops, the bed collapses; if it is too high, the powder may be blown out of the reactor.
Complexity of Control
Achieving the specific ratios of reducing gases requires sophisticated monitoring.
The system relies on the exact balance of nitrogen and reducing agents. Deviating from these ratios can disrupt the chemical equilibrium, leading to incomplete reduction or inefficient fuel use.
Making the Right Choice for Your Goal
To leverage a fluidized bed calcination system effectively, you must align your operational parameters with your output targets.
- If your primary focus is Reaction Speed: Prioritize the precise regulation of gas flow rates to maximize the kinetic energy and turbulence within the fluidization zone.
- If your primary focus is Energy Efficiency: Leverage the system's ability to convert hematite to magnetite at low temperatures by maintaining strict thermal controls to avoid overheating.
The ultimate success of suspension reduction lies in the rigorous management of gas-solid interaction to achieve high throughput with minimal thermal waste.
Summary Table:
| Key Feature | Functional Impact | Operational Benefit |
|---|---|---|
| Porous Quartz Plate | Even gas distribution | Prevents powder settling, ensures suspension |
| Suspended State | Maximized surface area | Faster reaction kinetics and chemical conversion |
| Thermal Uniformity | Efficient heat exchange | Low-temperature conversion of hematite to magnetite |
| Gas Flow Control | Precise nitrogen/reductant ratio | Maintains fluidization and chemical equilibrium |
Optimize Your Calcination Process with KINTEK
Unlock the full potential of your metallurgical research with KINTEK’s high-precision thermal solutions. Backed by expert R&D and world-class manufacturing, we provide high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems tailored to your exact specifications.
Whether you are refining iron ore powder or developing new material phases, our customizable lab high-temperature furnaces deliver the thermal uniformity and gas-regulation precision you need for successful suspension reduction.
Ready to elevate your lab's efficiency? Contact our experts today to discuss your unique project requirements.
Visual Guide
References
- Pengcheng Hou, Yongsheng Sun. Mechanism of effective iron extraction from rare earth-bearing iron ores by low-temperature suspension reduction method. DOI: 10.37190/ppmp/204110
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is a pyrolysis rotary kiln reactor and its function? A Complete Guide to Industrial Waste Conversion
- What are the typical rotation speeds for a rotary kiln and how do they affect material retention time? Optimize Your Kiln Performance
- What are the advantages of rotary tube furnaces in fuel compatibility? Boost Efficiency and Cut Costs
- How does the rotating design of the rotary tube sintering furnace improve heating uniformity? Achieve Consistent Results
- What is the significance of customizable rotation and process control in a rotary furnace? Unlock Precision and Efficiency in Thermal Processing
- What is the role of a Rotary Chemical Vapor Deposition (Rotary CVD) system? Optimize Hollow Silica Particle Coating
- What roles does a rotary cement kiln play in cement production? Uncover Its 4 Critical Functions
- What is the role of rotary tube furnaces in the energy sector? Boost Efficiency in Biomass and Battery Material Processing



















