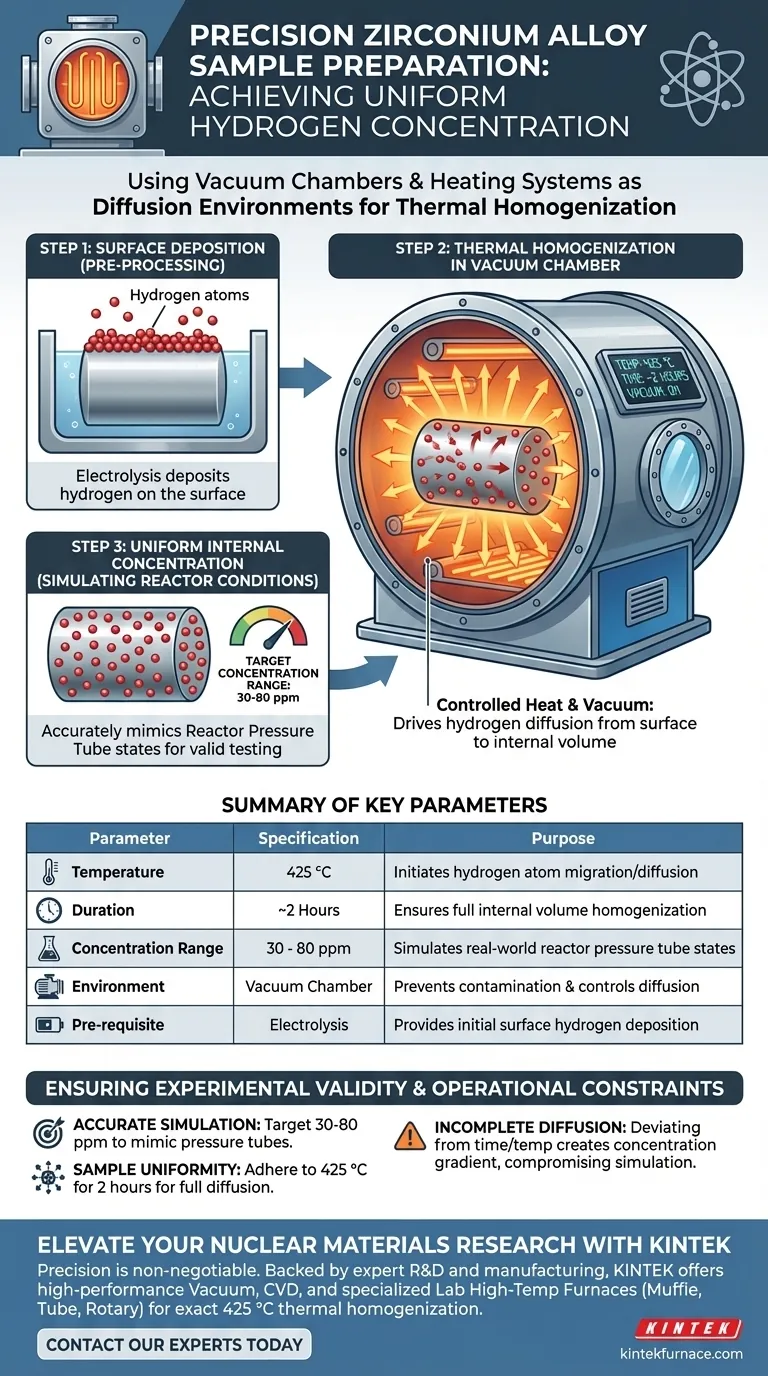
Vacuum chambers and heating systems function as precise diffusion environments used to prepare zirconium alloy samples. They facilitate the preparation process through thermal homogenization, specifically by heating samples to 425 °C for approximately two hours within a vacuum. This controlled environment drives hydrogen, originally deposited on the surface during electrolysis, to diffuse uniformly into the internal volume of the alloy.
The core purpose of this setup is to convert surface-level hydrogen into a uniform internal concentration. This ensures the material achieves specific hydrogen levels (typically 30-80 ppm) that accurately simulate the conditions of real-world reactor pressure tubes.
The Mechanism of Thermal Homogenization
Moving Hydrogen from Surface to Core
The process begins after the zirconium alloy has undergone electrolysis. At this stage, the hydrogen is primarily located on the surface of the material.
The vacuum chamber and heating system work together to initiate diffusion. By applying heat, the equipment provides the energy necessary for hydrogen atoms to migrate from the surface into the bulk of the alloy.
The Role of Controlled Heating
The specific temperature regime is critical for success. The system maintains the samples at 425 °C for a duration of approximately two hours.
This sustained thermal exposure ensures the diffusion is thorough. It prevents hydrogen from remaining localized at the surface, creating a homogeneous distribution throughout the sample.
Simulating Reactor Conditions
Achieving Specific Concentrations
Researchers use this method to target precise hydrogen concentrations. The system is capable of stabilizing levels in the 30-80 ppm range.
This range is not arbitrary; it is selected to mirror specific operational states. Control over these concentrations is vital for experimental validity.
Replicating Pressure Tube State
The ultimate goal of using vacuum chambers for this preparation is simulation. The resulting samples must reflect the actual state of reactor pressure tubes.
By achieving uniform distribution and specific concentration levels, researchers can reliably test how actual reactor components will behave under similar chemical conditions.
Operational Constraints and Considerations
Dependency on Pre-processing
It is important to note that this thermal treatment is a secondary step. It explicitly follows electrolysis, meaning the vacuum system is effective only if the initial surface deposition is performed correctly.
Time-Temperature Sensitivity
The process relies on a specific combination of time and temperature. Deviating from the 425 °C setpoint or shortening the two-hour window may result in incomplete diffusion.
Incomplete diffusion leads to a gradient of hydrogen concentration rather than a homogenized sample, which would compromise the accuracy of the simulation.
Ensuring Experimental Validity
To maximize the effectiveness of this preparation method for your specific research goals, consider the following:
- If your primary focus is accurate simulation: Ensure your target hydrogen concentration falls strictly within the 30-80 ppm range to mimic reactor pressure tubes.
- If your primary focus is sample uniformity: Strictly adhere to the two-hour duration at 425 °C to guarantee full diffusion from the surface to the internal volume.
By strictly controlling the thermal vacuum environment, you ensure the zirconium alloy samples provide a reliable baseline for nuclear reactor research.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Temperature | 425 °C | Initiates hydrogen atom migration/diffusion |
| Duration | ~2 Hours | Ensures full internal volume homogenization |
| Concentration Range | 30 - 80 ppm | Simulates real-world reactor pressure tube states |
| Environment | Vacuum Chamber | Prevents contamination and controls diffusion |
| Pre-requisite | Electrolysis | Provides initial surface hydrogen deposition |
Elevate Your Nuclear Materials Research with KINTEK
Precision is non-negotiable when simulating reactor pressure tube conditions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Vacuum, CVD, and specialized Lab High-Temp Furnaces designed to deliver the exact 425 °C thermal homogenization required for zirconium alloy preparation.
Whether you need customizable Muffle, Tube, or Rotary systems for uniform hydrogen diffusion or complex material synthesis, our equipment provides the stability and control your experiments demand.
Ready to achieve superior sample uniformity? Contact our experts today to find the perfect customized heating solution for your laboratory needs.
Visual Guide
References
- Alexandra Jinga, Mircea Ionuţ Petrescu. Evaluation of the Zirconium Hydride Morphology at the Flaws in the CANDU Pressure Tube Using a Novel Metric. DOI: 10.3390/app15020787
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What ceramic materials can be processed in vacuum heat treatment furnaces? Unlock High-Purity Processing for Advanced Ceramics
- What technical advantages do electric furnace systems offer for copper slag impoverishment? Maximize Your Metal Recovery
- What is the role of inert gas in a vacuum furnace? Unlock Rapid, Controlled Cooling for Superior Metallurgy
- Why is vertical stack loading superior to staggered stack loading in batch gas quenching? Optimize Gas Flow & Quality
- How is furnace brazing used in research and development? Unlock Precision Joining for Material Innovation
- Why is a high vacuum furnace necessary for the solution treatment of cold-rolled TNZTSF alloys? Prevent Oxidation.
- What are the benefits of vacuum heat treatment for workpieces? Enhance Precision and Durability
- What factors influence the price of vacuum furnaces? Key Drivers from Size to Automation



















