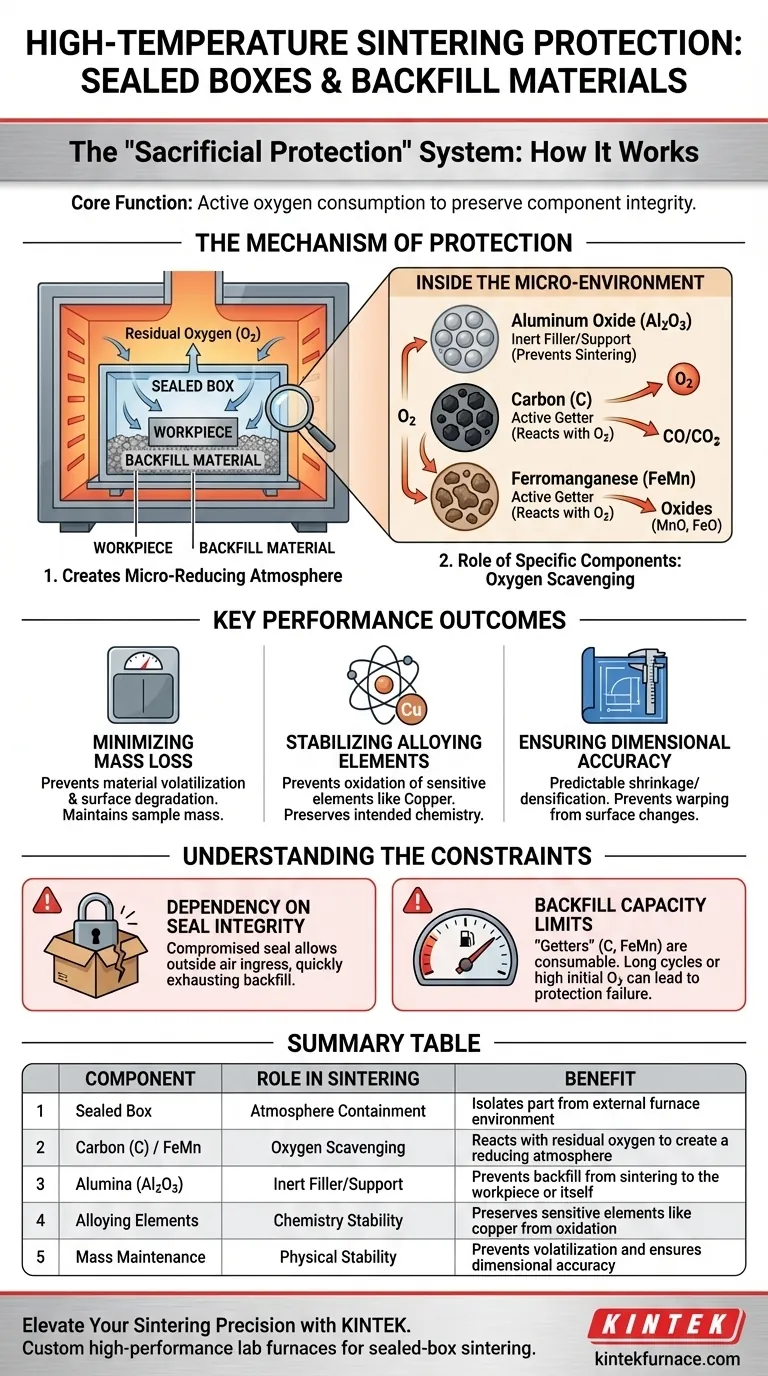
Sealed boxes combined with specific backfill materials function as a protective isolation system that chemically alters the sintering environment. By trapping a mixture of aluminum oxide, ferromanganese, and carbon powder around the workpiece, this setup generates a localized micro-reducing atmosphere that actively consumes oxygen before it can damage the part.
The core function of this system is "sacrificial protection." By using reactive backfill agents within a confined space, the process ensures that oxygen attacks the backfill material rather than the component, preserving the mass, chemistry, and dimensions of the final product.

The Mechanism of Protection
Creating a Micro-Reducing Atmosphere
The primary role of the sealed box is to physically contain the immediate atmosphere around the sintered part. Inside this enclosure, the backfill material—specifically a mix of Carbon (C) and Ferromanganese (FeMn)—acts as an oxygen scavenger.
These materials react with residual oxygen more readily than the workpiece does. This reaction effectively scrubs oxygen from the micro-environment, lowering the partial pressure of oxygen to safe levels.
The Role of Specific Components
The backfill mixture relies on a balance of inert and active ingredients. Aluminum Oxide (Al2O3) typically serves as the inert structural support or filler, preventing the backfill from sintering to itself or the part.
Meanwhile, the Carbon and Ferromanganese serve as the active "getters." They sacrifice themselves to neutralize oxidation potential, ensuring the atmosphere remains reducing rather than oxidizing.
Key Performance Outcomes
Minimizing Mass Loss
At high sintering temperatures, materials are prone to volatilization or surface degradation if exposed to uncontrolled atmospheres.
The sealed environment prevents this degradation. By consuming the oxygen, the backfill ensures that the material of the sample remains intact, significantly reducing mass loss during the thermal cycle.
Stabilizing Alloying Elements
Certain alloying elements, such as copper, are highly sensitive to oxidation. If oxygen is present, these elements react and drop out of the metallic solution, altering the material's properties.
The micro-reducing atmosphere specifically prevents the oxidation of these sensitive elements. This ensures the final alloy composition matches the intended design.
Ensuring Dimensional Accuracy
Chemical stability leads to physical stability. When oxidation and mass loss are prevented, the sintering process becomes more predictable.
This protection is critical for stabilizing the final dimensions of the material. It ensures that the part shrinks or densifies at a controlled rate, rather than warping due to surface chemistry changes.
Understanding the Constraints
Dependency on Seal Integrity
The effectiveness of this method relies entirely on the isolation provided by the box. If the seal is compromised, the finite amount of backfill material will be quickly exhausted by the ingress of outside air.
Backfill Capacity Limits
The "getter" materials (C and FeMn) are consumable. In a sealed environment, there is a limit to how much oxygen they can absorb before they are fully reacted.
If the sintering cycle is too long or the residual oxygen content is too high initially, the protection may fail midway through the process.
Applying This to Your Sintering Process
To maximize the quality of your powder metallurgy components, align your approach with your specific quality metrics:
- If your primary focus is Dimensional Precision: Utilize this sealed system to prevent surface degradation and mass loss, which are the primary causes of unpredictable warping and shrinkage.
- If your primary focus is Material Chemistry: Rely on the Ferromanganese and Carbon backfill to preserve sensitive alloying elements like copper, ensuring the mechanical properties remain consistent.
This method transforms the sintering atmosphere from a variable risk into a controlled tool for quality assurance.
Summary Table:
| Component | Role in Sintering | Benefit |
|---|---|---|
| Sealed Box | Atmosphere Containment | Isolates the part from the external furnace environment |
| Carbon (C) / FeMn | Oxygen Scavenging | Reacts with residual oxygen to create a reducing atmosphere |
| Alumina (Al2O3) | Inert Filler/Support | Prevents backfill from sintering to the workpiece or itself |
| Alloying Elements | Chemistry Stability | Preserves sensitive elements like copper from oxidation |
| Mass Maintenance | Physical Stability | Prevents volatilization and ensures dimensional accuracy |
Elevate Your Sintering Precision with KINTEK
Don't let oxidation compromise your metallurgy quality. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to accommodate sealed-box sintering and specific atmosphere requirements. Whether you are aiming for superior dimensional accuracy or stabilized alloy chemistry, our high-temperature lab furnaces provide the control you need.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Petko Naydenov. DETERMING THE COMPENSATING ACTION OF COPPER AFTER SINTERING OF POWDER METALLURGICAL STRUCTURAL STEELS. DOI: 10.17770/etr2025vol4.8439
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- Why are high-purity alumina crucibles preferred over quartz crucibles at 1873 K? Ensure Precision at Extreme Heat
- What is the function of a specifically designed annealing vessel in SVA? Enhance Your Film Crystallization Today
- Why is a high-purity graphite crucible typically chosen for the high-temperature vacuum carbothermic reduction of magnesium oxide?
- How are laboratory vacuum pumps utilized in 1T-TaS2 crystal preparation? Ensure Peak Sample Purity
- How do high-precision mass flow controllers contribute to studying the oxidation behavior of lignite?
- What are quartz tubes used for? Essential for High-Temperature, High-Purity Applications
- Why is a Mass Flow Controller (MFC) necessary for CDM testing? Ensure Precise Kinetic Data and Catalyst Performance
- What role does a PTFE-lined high-pressure autoclave play in synthesis of ZnO nanorods? Key Benefits & Growth Factors



















