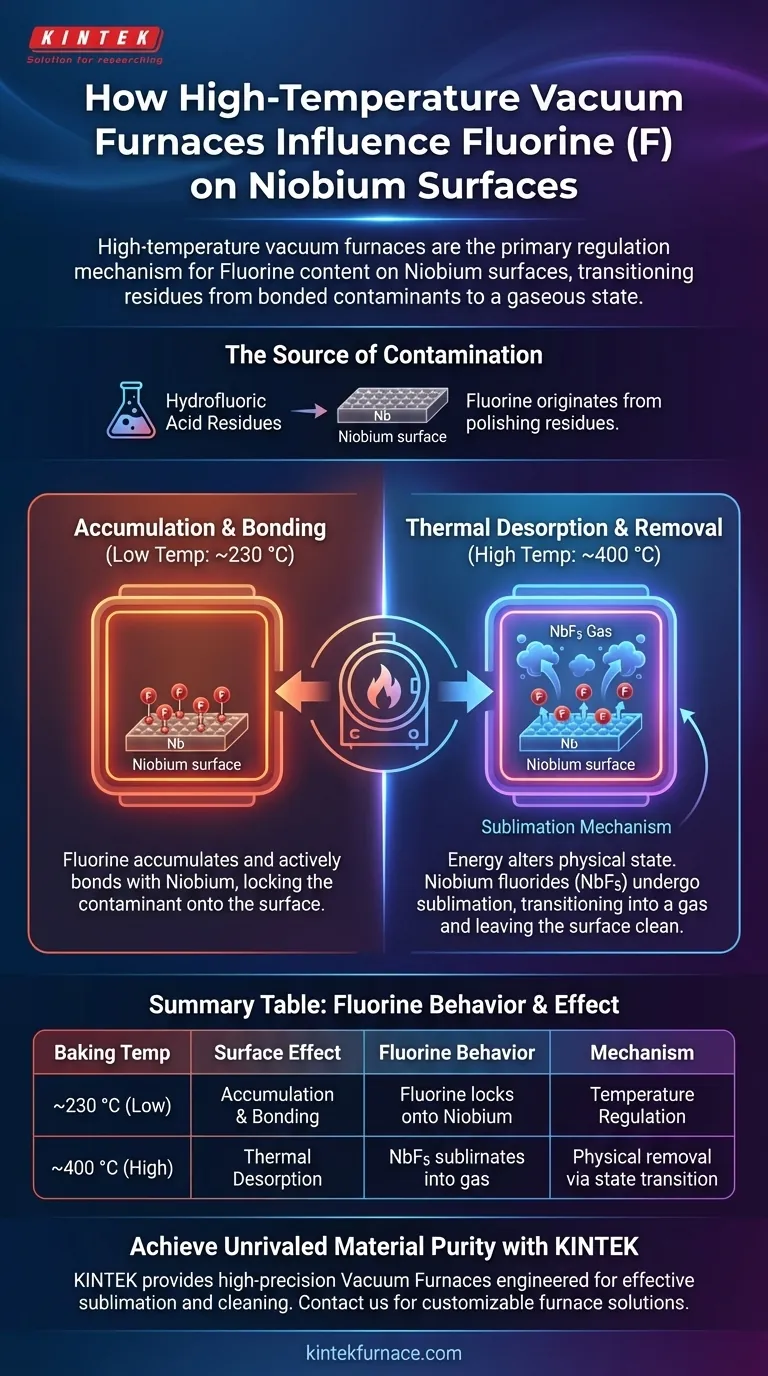
High-temperature vacuum furnaces act as the primary regulation mechanism for controlling Fluorine content on Niobium surfaces. By manipulating thermal conditions, these furnaces transition Fluorine residues from a bonded surface contaminant into a gaseous state, effectively stripping them away during the baking process.
While chemical polishing leaves behind Fluorine residues that bond to Niobium at lower temperatures, the vacuum furnace solves this by raising the environment to a threshold where these compounds thermally desorb. This process relies on sublimation to physically remove contaminants rather than just chemically altering them.

The Source of Surface Contamination
Hydrofluoric Acid Residues
The Fluorine found on Niobium surfaces is not inherent to the material itself. It originates from hydrofluoric acid residues left behind during the chemical polishing phase of manufacturing.
The Role of the Furnace
The vacuum furnace serves as the critical control point for managing these residues. It determines whether the Fluorine remains as a surface impurity or is successfully eliminated.
The Temperature-Dependent Mechanism
Accumulation at Lower Temperatures (~230 °C)
Temperature selection is the deciding factor in surface chemistry. At lower baking temperatures, specifically around 230 °C, the furnace does not remove the Fluorine.
Bonding Behavior
Instead of leaving the surface at this temperature, Fluorine accumulates. It actively bonds with the Niobium, effectively locking the contaminant onto the material rather than cleaning it.
Desorption at Higher Temperatures (~400 °C)
To achieve cleaning, the furnace must reach higher thermal thresholds. At approximately 400 °C, the energy provided by the furnace alters the physical state of the surface compounds.
The Sublimation of NbF5
At this elevated temperature, Niobium fluorides (specifically NbF5) undergo thermal desorption or sublimation. This means the solid compounds transition directly into a gas, detaching from the Niobium surface and leaving it clean.
Understanding the Trade-offs
The Risk of Insufficient Heat
The primary pitfall in this process is failing to reach the desorption threshold. If the furnace operates solely in the lower range (near 230 °C), you risk consolidating the contamination rather than removing it.
Process Precision
This creates a binary outcome based on thermal precision. You are either bonding contaminants to the surface or sublimating them away; there is little middle ground in terms of chemical elimination.
Making the Right Choice for Your Goal
To optimize the quality of your Niobium surfaces, you must align your temperature settings with your desired chemical outcome.
- If your primary focus is retention and bonding: Operate at lower temperatures (~230 °C) to allow Fluorine to accumulate and bond with the Niobium structure.
- If your primary focus is surface purity: Elevate the process to ~400 °C to trigger the sublimation of NbF5 and effectively strip Fluorine residues.
Control the temperature precisely to dictate whether Fluorine becomes a permanent fixture or a removed byproduct.
Summary Table:
| Baking Temperature | Surface Chemical Effect | Fluorine Behavior |
|---|---|---|
| ~230 °C (Low) | Accumulation & Bonding | Fluorine locks onto Niobium structure |
| ~400 °C (High) | Thermal Desorption | NbF5 sublimates into a gaseous state |
| Mechanism | Temperature Regulation | Physical removal via state transition |
Achieve Unrivaled Material Purity with KINTEK
Don't let surface contaminants compromise your Niobium processing. KINTEK provides high-precision Vacuum Furnaces engineered to deliver the exact thermal thresholds required for effective sublimation and cleaning.
Backed by expert R&D and manufacturing, we offer a comprehensive range of customizable systems—including Muffle, Tube, Rotary, Vacuum, and CVD furnaces—tailored to meet your specific laboratory or industrial high-temperature needs.
Ready to optimize your thermal processes? Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Alena Prudnikava, Jens Knobloch. <i>In-situ</i> synchrotron x-ray photoelectron spectroscopy study of medium-temperature baking of niobium for SRF application. DOI: 10.1088/1361-6668/ad4825
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What are the advantages of conducting heat treatment in a vacuum environment? Achieve Superior Material Control and Quality
- How does a circulating water cooling system contribute to the stable operation of high-temp vacuum furnaces? | KINTEK
- What are the advantages of using vacuum-based processing for Co3O4@CNT composites? Preserve 3D Architecture Today
- Why is a vacuum heat treatment furnace required for IN718-CuCrZr? Maximize Bimetallic Bond Strength
- What is the function of the heating chamber in a drop-bottom quench furnace? Ensure Precise Heat Treatment for Superior Metallurgy
- What is the process of vacuum annealing? Achieve Superior Material Purity and Performance
- What is a hot wall vacuum furnace design? Discover Its Key Benefits and Applications
- Why is vacuum brazing considered a clean process? Achieve Oxide-Free, Flux-Free Metal Joining



















