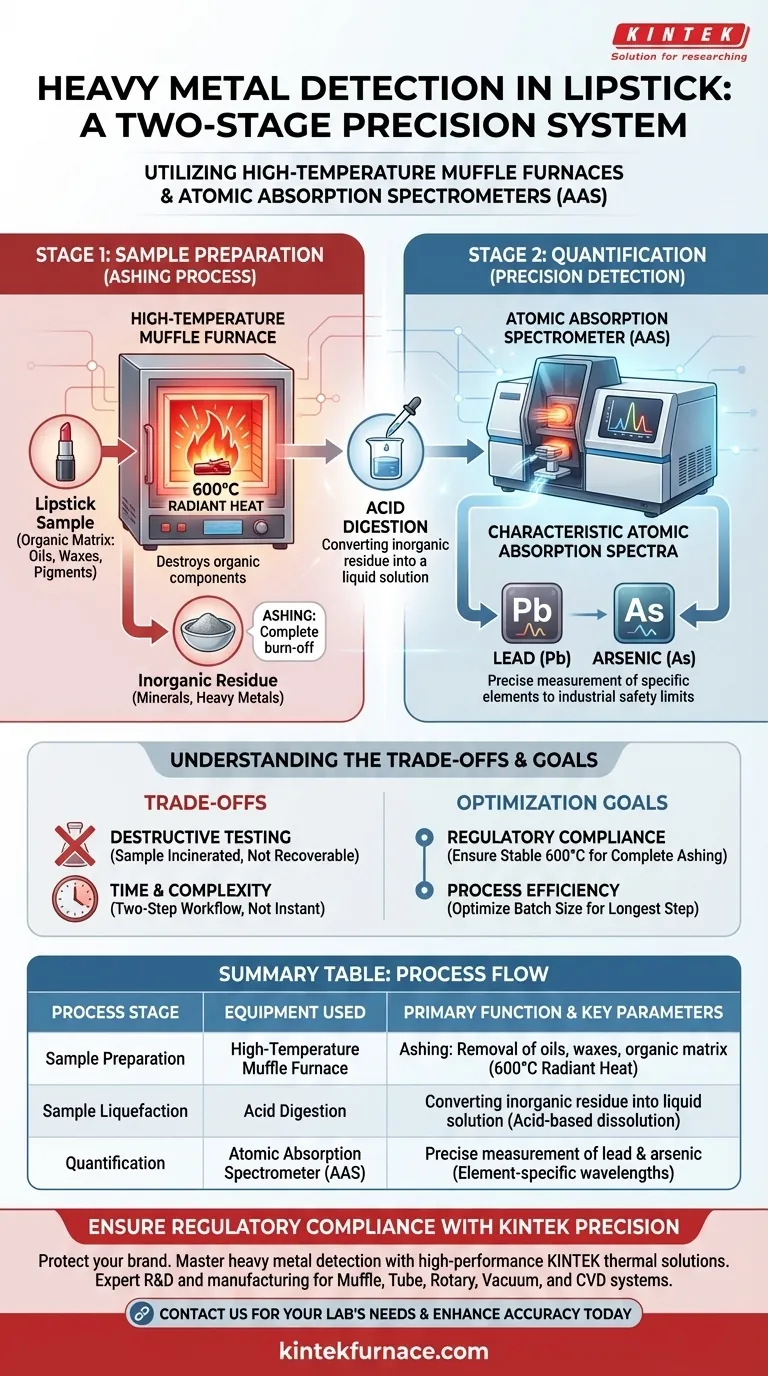
High-temperature muffle furnaces and atomic absorption spectrometers (AAS) function as a critical two-stage system to detect toxic heavy metals in lipstick products. The muffle furnace is first used to perform an "ashing" process at 600°C, which completely burns off the cosmetic's organic waxes and oils; the AAS then analyzes the remaining acid-digested residue to precisely quantify hazardous elements like lead and arsenic.
Core Takeaway: Effective heavy metal detection requires separating the target metals from the complex cosmetic ingredients. The muffle furnace acts as the preparation tool to destroy the organic matrix, while the AAS serves as the precision instrument to verify that the remaining trace metals fall within safe industrial limits.

Preparing the Sample: The Role of the Muffle Furnace
Removing the Organic Matrix
Lipstick is composed of a dense mixture of oils, waxes, and pigments. Before analysis can occur, this organic matrix must be removed to prevent interference with detection equipment.
The Ashing Process
To achieve this, samples are placed in a high-temperature muffle furnace. The furnace creates a controlled environment utilizing radiant heat transfer to bring the sample to 600°C.
Creating a Testable Residue
At this extreme temperature, the organic components combust and vaporize, leaving behind only the inorganic minerals and metals. This process, known as ashing, results in a residue that contains the heavy metals in a form ready for chemical processing.
Quantifying Toxicity: The Role of the AAS
Acid Digestion
Once the ashing process is complete, the remaining inorganic residue is not tested directly as a solid. It is subjected to acid digestion, turning the ash into a liquid solution suitable for the spectrometer.
Characteristic Atomic Absorption
The Atomic Absorption Spectrometer (AAS) analyzes this solution by utilizing the characteristic atomic absorption spectra of specific elements. Every element absorbs light at a unique wavelength, acting like a fingerprint.
Precision Detection
The AAS measures how much light is absorbed by the sample at specific wavelengths. This allows it to detect the exact levels of harmful heavy metals, specifically lead and arsenic, ensuring the product complies with strict industrial safety limits.
Understanding the Trade-offs
Destructive Testing
This method is inherently destructive. Because the sample is incinerated at 600°C and then dissolved in acid, the product cannot be recovered. This makes it suitable for batch quality control, but not for testing finished goods intended for sale.
Time and Complexity
The process is not instantaneous. It requires a distinct two-step workflow—thermal decomposition followed by chemical analysis. This is more time-consuming than surface scanning methods but offers significantly higher accuracy for deep-matrix analysis.
Making the Right Choice for Your Goal
To apply this workflow effectively, consider your specific compliance needs:
- If your primary focus is Regulatory Compliance: Ensure your muffle furnace is calibrated to maintain a stable 600°C to guarantee the complete removal of the organic matrix without volatilizing the target metals.
- If your primary focus is Process Efficiency: Optimize the batch size for the ashing process, as the furnace is the longest step in the detection cycle.
By rigorously burning away organic interference and utilizing spectral precision, you ensure the highest standard of consumer safety.
Summary Table:
| Process Stage | Equipment Used | Primary Function | Key Parameters |
|---|---|---|---|
| Sample Preparation | High-Temperature Muffle Furnace | Ashing: Removal of oils, waxes, and organic matrix | 600°C Radiant Heat |
| Sample Liquefaction | Acid Digestion | Converting inorganic residue into a liquid solution | Acid-based dissolution |
| Quantification | Atomic Absorption Spectrometer (AAS) | Precise measurement of lead and arsenic levels | Element-specific wavelengths |
Ensure Regulatory Compliance with KINTEK Precision
Protect your brand and consumers by mastering the complex process of heavy metal detection. KINTEK provides high-performance, customizable thermal solutions designed for rigorous laboratory workflows. Backed by expert R&D and manufacturing, we offer high-stability Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for precise ashing and material analysis.
Ready to enhance your lab's accuracy and process efficiency? Contact us today to find the perfect high-temperature furnace for your unique testing needs.
Visual Guide
References
- Uma There, Vibha Kapoor. Development and assessment of red sandalwood, cocoa powder, and beetroot pigmented lipsticks. DOI: 10.33545/26646781.2025.v7.i8a.303
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does the muffle furnace prevent run-away conditions? Ensure Safe, Reliable High-Temperature Operations
- What furnace features ensure high-quality firing? Achieve Uniform Sintering & Superior Results
- What is the purpose of ashing in a muffle furnace? Unlock Material Purity and Quality Insights
- What is a muffle furnace and its primary use? Discover High-Temp, Contamination-Free Heating Solutions
- How is a muffle furnace utilized in ash testing? Achieve Accurate Mineral Analysis for Your Lab
- Why is precise temperature control critical when sintering 13-93 bioactive glass? Expert Thermal Management Guide
- What applications does a muffle furnace have in coal quality analysis? Essential for Precise Coal Testing
- What industries commonly use industrial muffle furnaces? Unlock Precision Heating for Diverse Sectors



















