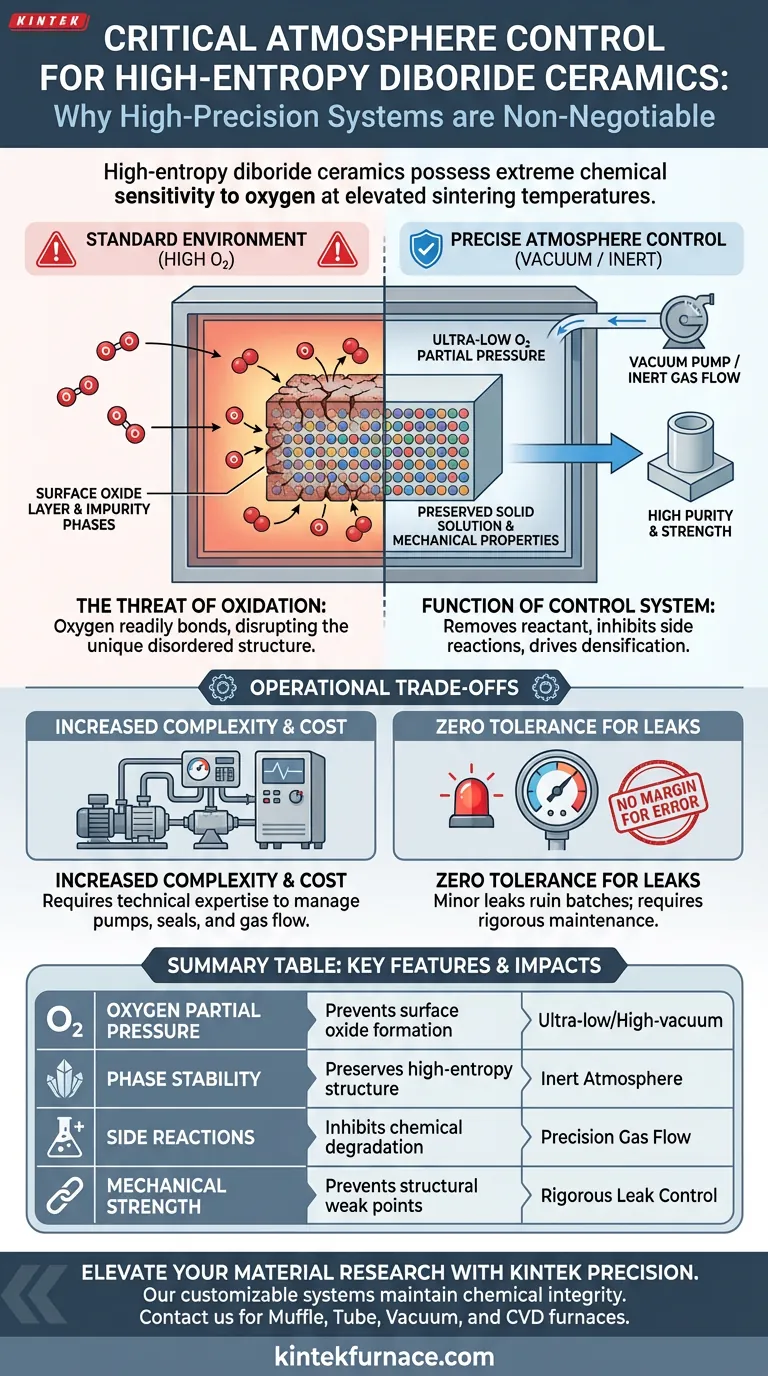
High-entropy diboride ceramics demand precise atmosphere control because they possess an extreme chemical sensitivity to oxygen at the elevated temperatures required for sintering. Without a high-precision vacuum or inert atmosphere system, even trace amounts of oxygen will react with the material, creating unwanted surface oxides and impurity phases. This equipment provides the critical environmental isolation necessary to inhibit oxidative side reactions and preserve the material's intended properties.
The furnace system acts as a protective chemical barrier. By maintaining ultra-low oxygen partial pressure, it prevents the degradation of the high-entropy solid solution phase, ensuring the final ceramic retains its high purity and mechanical strength.

The Chemistry of High-Temperature Sensitivity
The Threat of Oxidation
High-entropy diboride ceramics are extremely sensitive to oxidation when heated.
In standard environments, high temperatures accelerate chemical reactions. If oxygen is present, the ceramic components will readily bond with it rather than sintering together as intended.
Formation of Impurities
Contact with oxygen leads to the immediate formation of surface oxide layers.
These layers are not just cosmetic defects; they penetrate the material matrix. This introduces impurity phases that fundamentally alter the ceramic's composition.
Disrupting the Solid Solution
The performance of these ceramics relies on a specific high-entropy solid solution phase.
Oxidation disrupts this phase. It pulls elements out of the solution to form oxides, effectively breaking the unique disordered structure that gives these materials their superior qualities.
The Function of the Control System
Lowering Oxygen Partial Pressure
The primary role of the furnace's vacuum or atmosphere control is to maintain extremely low oxygen partial pressure.
By removing oxygen molecules from the chamber, the system removes the reactant necessary for oxidation to occur.
Inhibiting Side Reactions
Precision control inhibits oxidative side reactions.
This ensures that the thermodynamic energy in the furnace drives densification (sintering) rather than chemical degradation.
Preserving Mechanical Properties
Impurities caused by oxidation act as structural weak points.
By preventing these impurities, the control system prevents the degradation of mechanical properties. This ensures the final product achieves the hardness and durability expected of high-entropy ceramics.
Understanding the Operational Trade-offs
Increased Equipment Complexity
High-precision vacuum systems significantly increase the complexity and cost of the sintering setup.
Operators must manage pumps, seals, and gas flow controllers, requiring a higher level of technical expertise than standard air sintering requires.
Zero Tolerance for Leaks
The sensitivity of these materials means there is no margin for error.
A minor seal failure or a lapse in inert gas purity can ruin an entire batch. The system requires rigorous maintenance and monitoring to ensure the atmosphere remains compromised.
Making the Right Choice for Your Goal
To ensure the success of your sintering process, align your equipment choice with your specific material requirements:
- If your primary focus is maximizing phase purity: Prioritize a furnace with a high-vacuum system to achieve the lowest possible oxygen partial pressure.
- If your primary focus is process consistency: Ensure your inert atmosphere control system features precise flow regulation to maintain a stable environment throughout the heating cycle.
Atmosphere control is not merely a feature of the furnace; it is the fundamental enabler that allows high-entropy diboride ceramics to exist without degrading.
Summary Table:
| Key Feature | Impact on High-Entropy Ceramics | Requirement |
|---|---|---|
| Oxygen Partial Pressure | Prevents surface oxide and impurity phase formation | Ultra-low/High-vacuum |
| Phase Stability | Preserves high-entropy solid solution structure | Inert Atmosphere |
| Side Reactions | Inhibits chemical degradation during densification | Precision Gas Flow |
| Mechanical Strength | Prevents structural weak points and brittleness | Rigorous Leak Control |
Elevate Your Material Research with KINTEK Precision
High-entropy ceramics demand an environment where there is no margin for error. KINTEK provides the high-precision technology needed to maintain the chemical integrity of your most sensitive materials. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as other lab high-temp furnaces, all fully customizable to meet your specific sintering requirements.
Don't let oxidation compromise your results. Contact us today to find the perfect customizable solution for your lab!
Visual Guide
References
- Yajun Lv, Weizhun Jin. Preparation and Properties of Porous Concrete Based on Geopolymer of Red Mud and Yellow River Sediment. DOI: 10.3390/ma17040923
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
People Also Ask
- How does vacuum sintering improve surface finish? Achieve Superior, Oxide-Free Results
- What are the key components of a vacuum heat treatment furnace? Discover Precision Metallurgy Solutions
- What is the hot zone in a vacuum furnace? Key Components and Performance Insights
- What are the main advantages of continuous furnaces? Boost Efficiency and Cut Costs in Mass Production
- What heating elements are used in laboratory vacuum furnaces and their temperature ranges? Optimize Your High-Temp Processes
- How does a chiller protect the vacuum furnace itself? Extend Equipment Life with Effective Cooling
- What role does a vacuum annealing furnace play in the final heat treatment of Ti-5Al-2.5Sn-0.2C alloys? Master Carbon Management
- How does a vacuum heating system contribute to the resin modification? Enhance Density & Chemical Purity



















