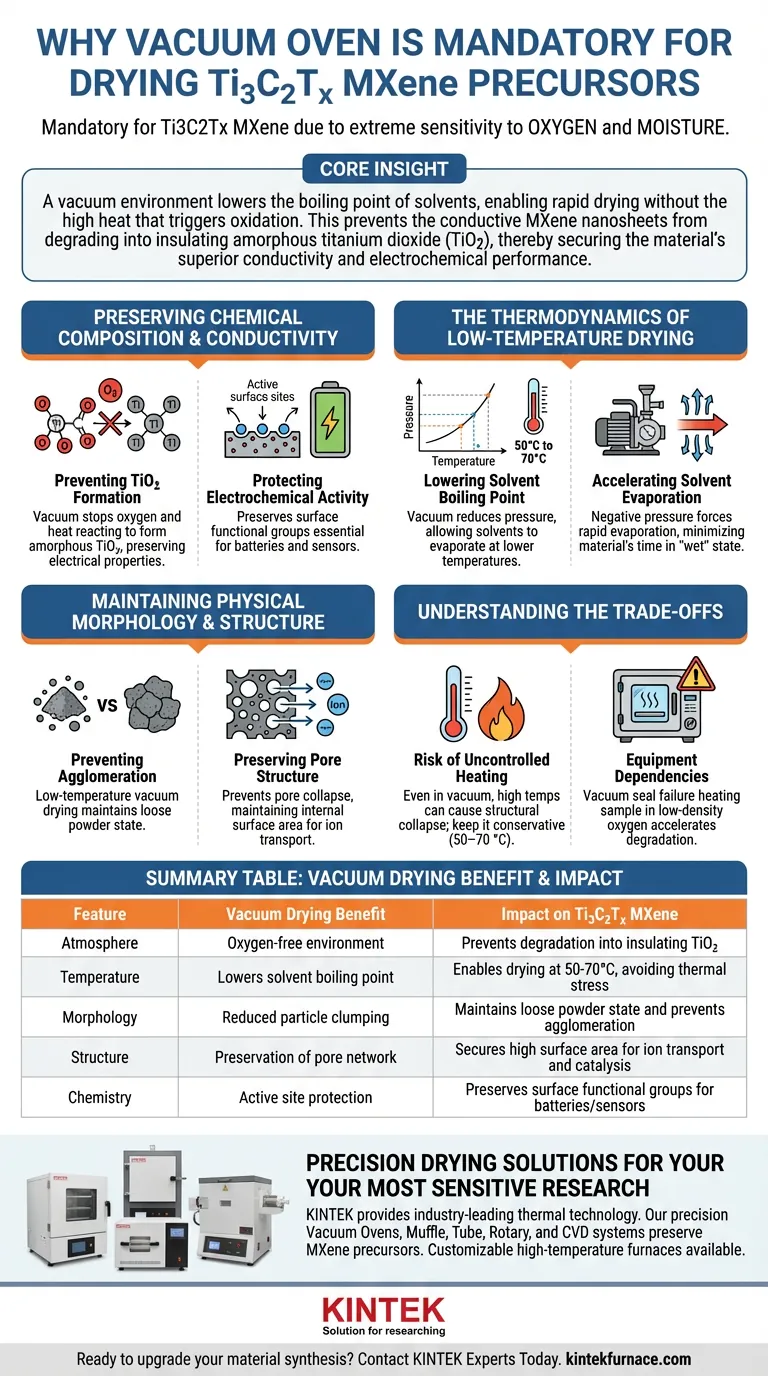
The mandatory use of a vacuum oven for Ti3C2Tx MXene precursors is dictated by the material's extreme sensitivity to oxygen and moisture. Ti3C2Tx is a reactive two-dimensional material that degrades rapidly when exposed to air, particularly at elevated temperatures. A vacuum oven provides a controlled, oxygen-free environment that allows for the removal of solvents at significantly lower temperatures, preserving the material's critical electrical and structural properties.
Core Insight A vacuum environment lowers the boiling point of solvents, enabling rapid drying without the high heat that triggers oxidation. This prevents the conductive MXene nanosheets from degrading into insulating amorphous titanium dioxide (TiO2), thereby securing the material's superior conductivity and electrochemical performance.
Preserving Chemical Composition and Conductivity
The primary danger to Ti3C2Tx MXene during synthesis is thermal oxidative degradation. The drying phase is the most vulnerable step in the process, and the vacuum oven is the specific engineering control used to mitigate this risk.
Preventing the Formation of TiO2
When MXene is dried in a standard atmosphere, the combination of oxygen and heat causes the titanium atoms in the lattice to react.
This leads to the formation of amorphous titanium dioxide (TiO2). Since TiO2 is a semiconductor with significantly lower conductivity than pure MXene, this transformation destroys the very electrical properties you are trying to engineer.
Protecting Electrochemical Activity
Beyond simple conductivity, the specific surface chemistry of MXene defines its utility in batteries and sensors.
Vacuum drying prevents the material from reacting with environmental oxygen, preserving the integrity of its surface functional groups. Maintaining these active sites is essential for high-performance applications like catalysis and energy storage.
The Thermodynamics of Low-Temperature Drying
The physical advantage of a vacuum oven lies in its ability to manipulate the relationship between pressure and temperature.
Lowering the Solvent Boiling Point
By reducing the environmental pressure, a vacuum oven allows water, ethanol, and other solvents to boil and evaporate at temperatures far below their standard boiling points (e.g., drying at 50°C to 70°C).
This allows you to achieve a completely dry powder without ever exposing the precursor to the thermal stress usually required to drive off moisture.
Accelerating Solvent Evaporation
Despite the lower temperatures, the drying process is often faster in a vacuum.
The negative pressure environment forces rapid evaporation. This efficiency minimizes the time the material spends in a "wet" state, further reducing the window of opportunity for chemical degradation.
Maintaining Physical Morphology and Structure
The method of drying dictates the final physical arrangement of the nanosheets. A vacuum oven ensures the structural architecture remains intact.
Preventing Agglomeration
Drying at high temperatures in ambient air often causes precursors to clump together.
Vacuum drying at lower temperatures helps maintain the precursor powder in a loose state. This prevents severe agglomeration, ensuring the individual nanosheets remain distinct rather than fusing into a dense, unusable block.
Preserving Pore Structure
For applications involving ion transport, such as supercapacitors, the internal pore structure is vital.
Vacuum drying helps prevent the collapse of the support pores and inhibits pore closure. This maintains a well-developed internal surface area, which is critical for allowing ions to move freely through the material.
Understanding the Trade-offs
While vacuum drying is superior for MXene, it requires precise control to be effective.
The Risk of uncontrolled Heating
Even in a vacuum, temperature matters. While the primary reference notes drying at 110 °C is possible, many supplementary protocols suggest lower temperatures (50–70 °C) are safer.
If the temperature is set too high—even without oxygen—you risk structural collapse or localized overheating. The vacuum facilitates drying, but the temperature setting must still be conservative to protect the nanosheets.
Equipment Dependencies
Unlike a standard convection oven, a vacuum oven introduces the variable of pressure stability.
If the vacuum seal fails during the process, you are effectively heating the sample in a low-density oxygen atmosphere, which can accelerate degradation faster than in ambient conditions. Constant monitoring of pressure levels is required.
Making the Right Choice for Your Goal
The vacuum oven is not just a drying tool; it is a preservation device for your precursor's properties.
- If your primary focus is Electrical Conductivity: You must use vacuum drying to strictly prevent the oxidation of Titanium into TiO2, which would insulate your material.
- If your primary focus is Porosity and Surface Area: You rely on the vacuum to allow low-temperature evaporation, which prevents the pore collapse associated with high-heat drying.
- If your primary focus is Process Efficiency: You utilize the vacuum to accelerate the removal of difficult solvents like water or ethanol without resorting to damaging temperatures.
By decoupling temperature from evaporation, the vacuum oven allows you to dry MXene precursors aggressively without compromising their delicate chemical structure.
Summary Table:
| Feature | Vacuum Drying Benefit | Impact on Ti3C2Tx MXene |
|---|---|---|
| Atmosphere | Oxygen-free environment | Prevents degradation into insulating TiO2 |
| Temperature | Lowers solvent boiling point | Enables drying at 50-70°C, avoiding thermal stress |
| Morphology | Reduced particle clumping | Maintains loose powder state and prevents agglomeration |
| Structure | Preservation of pore network | Secures high surface area for ion transport and catalysis |
| Chemistry | Active site protection | Preserves surface functional groups for batteries/sensors |
Precision Drying Solutions for Your Most Sensitive Research
Don't let oxidation compromise your high-performance materials. KINTEK provides industry-leading thermal technology backed by expert R&D and manufacturing. Our precision Vacuum Ovens, Muffle, Tube, Rotary, and CVD systems are designed to preserve the delicate chemical integrity of MXene precursors and other reactive materials.
Whether you need a standard setup or a fully customizable high-temperature furnace for your unique lab requirements, KINTEK delivers the control and stability your research demands.
Ready to upgrade your material synthesis?
Visual Guide
References
- Minghua Chen, Kun Liang. Engineering Ti3C2-MXene Surface Composition for Excellent Li+ Storage Performance. DOI: 10.3390/molecules29081731
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What is the function of a fixed-bed catalytic reactor in ex situ CHP? Optimize Your Bio-oil Quality Today
- What is the function of a laboratory vacuum drying oven for Fe-N-C catalysts? Preserve Nanoporous Structure
- What is the purpose of the two-step heat treatment process? Optimize Zirconolite-Based Glass-Ceramic Matrices
- What is the function of a vacuum drying oven in CMS synthesis? Ensure High-Purity Precursor Integrity
- Why is precise heating rate control necessary? Master Activated Carbon Heat Treatment with KINTEK
- What is the significance of temperature control precision in high-temperature furnaces for carbon-doped titanium dioxide?
- What advantages does AlMe2iPrO (DMAI) offer over Trimethylaluminum (TMA)? Achieve Superior Area Selectivity
- What is the necessity of preheating reinforcement materials? Eliminate Defects in Aluminum Alloys



















