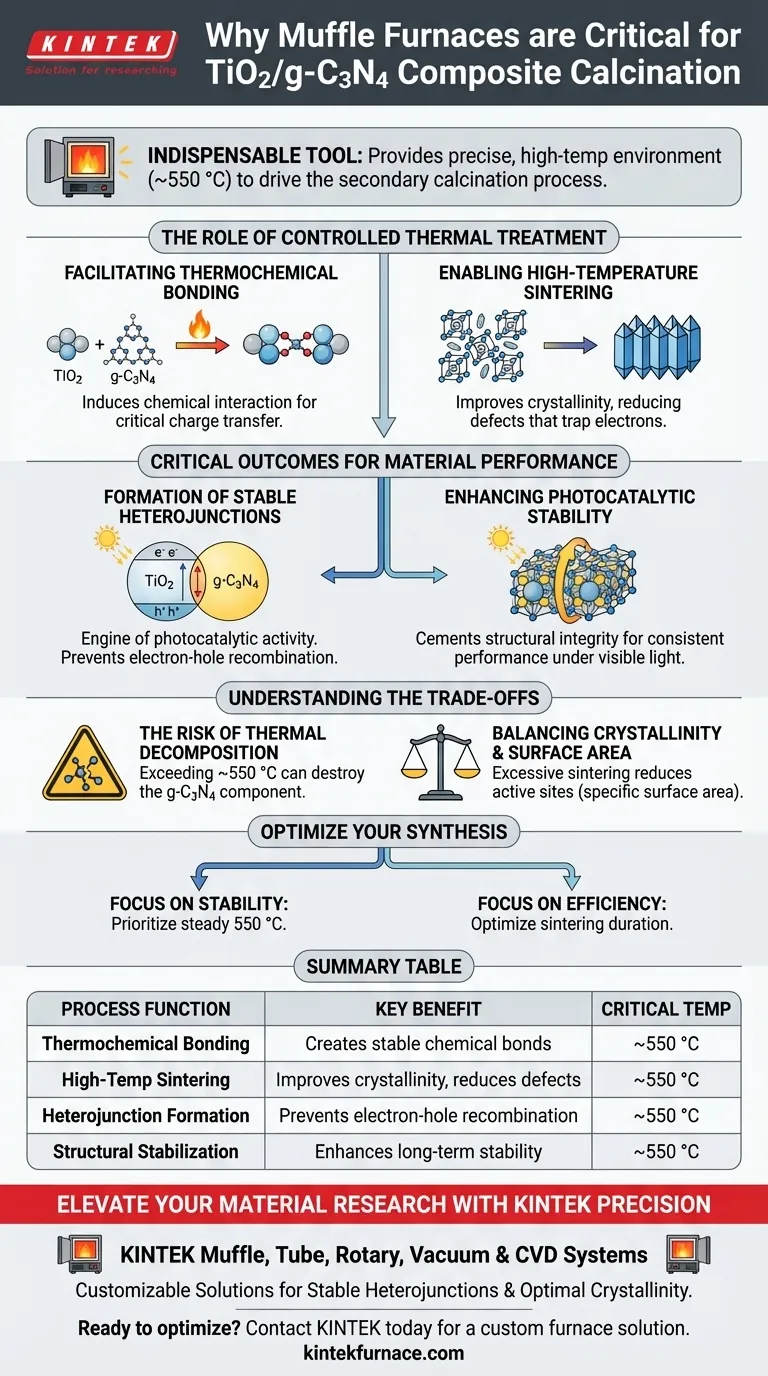
The use of a Muffle Furnace is indispensable for the successful synthesis of high-performance TiO2/g-C3N4 composite materials. It provides the precise, high-temperature environment—specifically around 550 °C—required to drive the secondary calcination process, transforming a simple mixture of precursors into a unified, chemically bonded composite.
The core function of the Muffle Furnace in this context is to induce thermochemical bonding and high-temperature sintering. This controlled thermal treatment creates stable heterojunction structures and improves crystallinity, which are absolute prerequisites for the material's photocatalytic stability and performance under visible light.

The Role of Controlled Thermal Treatment
Facilitating Thermochemical Bonding
The primary challenge in creating a composite is ensuring the two distinct materials interact at a chemical level rather than remaining a physical mixture.
The Muffle Furnace provides the necessary energy to induce thermochemical bonding between the TiO2 and g-C3N4 components. This bonding is critical for charge transfer between the materials during photocatalysis.
Enabling High-Temperature Sintering
Achieving the correct crystal structure requires sustained, uniform heat that standard ovens cannot provide.
Through high-temperature sintering, the furnace ensures the material achieves the necessary crystallinity. Improved crystallinity reduces defects that can trap electrons, thereby enhancing the overall efficiency of the material.
Critical Outcomes for Material Performance
Formation of Stable Heterojunctions
The interface where TiO2 meets g-C3N4 is known as the heterojunction. This is the engine of the composite's photocatalytic activity.
The precise 550 °C environment provided by the Muffle Furnace solidifies these heterojunction structures. A stable heterojunction ensures that electron-hole pairs generated by light are effectively separated, preventing them from recombining instantly.
Enhancing Photocatalytic Stability
A material may perform well initially, but structural weakness will lead to rapid degradation under operating conditions.
The thermal treatment cements the structural integrity of the composite. This process significantly enhances the material's photocatalytic stability, allowing it to maintain performance over time even under continuous visible light exposure.
Understanding the Trade-offs
The Risk of Thermal Decomposition
While high heat is necessary for bonding, g-C3N4 is an organic-like semiconductor that can degrade if temperatures are uncontrolled.
If the furnace temperature exceeds the optimal range (e.g., significantly above 550 °C), the g-C3N4 component may decompose entirely. This would destroy the heterojunction and leave behind only TiO2, negating the purpose of the composite.
Balancing Crystallinity and Surface Area
Sintering improves crystallinity, which helps electrical performance, but it can also lead to particle growth.
Excessive sintering times in the furnace can cause the particles to merge too aggressively. This reduces the specific surface area, potentially limiting the number of active sites available for chemical reactions.
Making the Right Choice for Your Goal
To optimize your TiO2/g-C3N4 synthesis, align your furnace parameters with your specific performance targets:
- If your primary focus is Long-Term Stability: Prioritize a steady 550 °C calcination to ensure robust thermochemical bonding and heterojunction solidity.
- If your primary focus is Charge Transfer Efficiency: Focus on the sintering duration to maximize crystallinity without inducing thermal decomposition of the g-C3N4 component.
Precision in thermal treatment is the bridge between a simple powder mixture and a functional, high-stability photocatalyst.
Summary Table:
| Process Function | Key Benefit | Critical Temperature |
|---|---|---|
| Thermochemical Bonding | Creates stable chemical bonds between TiO2 and g-C3N4 | ~550 °C |
| High-Temp Sintering | Improves crystallinity and reduces material defects | ~550 °C |
| Heterojunction Formation | Prevents electron-hole recombination for efficiency | ~550 °C |
| Structural Stabilization | Enhances long-term photocatalytic stability | ~550 °C |
Elevate Your Material Research with KINTEK Precision
Precise thermal control is the difference between a failed mixture and a high-performance composite. KINTEK provides industry-leading Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of TiO2/g-C3N4 synthesis. Backed by expert R&D and advanced manufacturing, our lab high-temp furnaces are fully customizable to your unique research needs, ensuring stable heterojunctions and optimal crystallinity every time.
Ready to optimize your calcination process? Contact KINTEK today for a custom furnace solution.
Visual Guide
References
- Rahil Azhar, W.I. Nawawi. Effect of Different Preparation Approaches on Pt-Modified TiO2/g-C3N4 for Effective Photocatalytic Degradation of RR4 Dye Under Visible Light. DOI: 10.24191/srj.v22i2.31241
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are some additional applications of muffle furnaces? Discover Versatile Uses in Labs and Industry
- What are the main applications of box type electric furnaces? Unlock Precision in Material Processing
- How are box type electric furnaces applied in electronic component manufacturing? Unlock Precision Thermal Processing
- What is the reputation of box furnaces in terms of quality and reliability? Trusted for Decades in High-Stakes Applications
- What are the specific functions of a muffle furnace in PLxZSH ceramic treatment? Optimize Debinding & Sintering
- What are the standard specifications for Box Furnaces? Key Components for Precision and Efficiency
- What role does a high-temperature muffle furnace play in the component analysis of Moringa oleifera seeds?
- How should metal materials with grease be handled in a muffle furnace? Prevent Damage and Extend Furnace Life



















