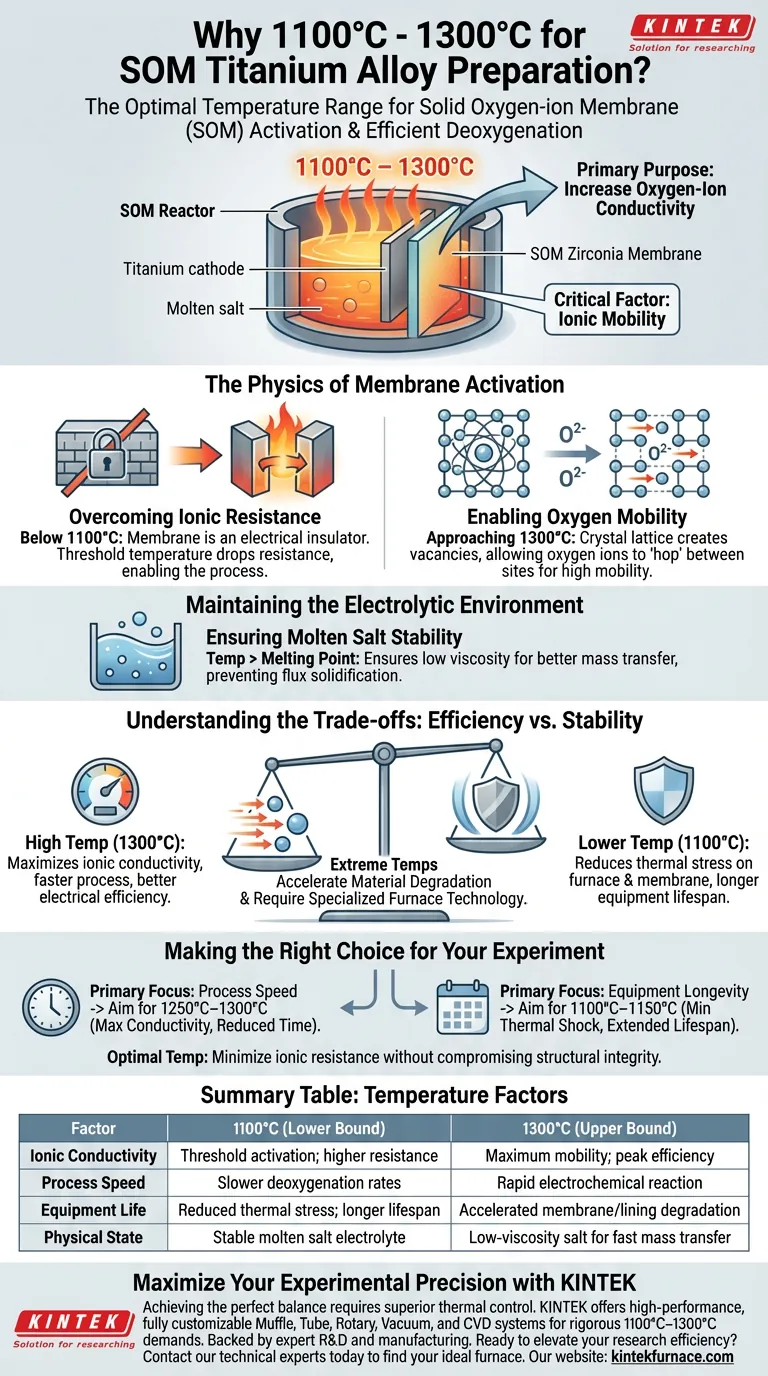
The temperature range of 1100°C to 1300°C is selected specifically to overcome the activation energy barrier of the Solid Oxygen-ion Membrane (SOM). While this heat ensures the molten salt electrolyte remains liquid, its primary technical purpose is to drastically increase the oxygen-ion conductivity of the membrane material, ensuring efficient deoxygenation of the titanium.
The critical factor driving this temperature selection is ionic mobility. At temperatures below 1100°C, the ceramic membrane acts as an insulator; only within this high-temperature window does it become sufficiently conductive to facilitate the electrochemical separation of oxygen from the alloy.

The Physics of Membrane Activation
Overcoming Ionic Resistance
The core of the SOM process is the solid membrane, typically composed of zirconia-based ceramics. At standard temperatures, these materials are electrically resistive.
To function as an electrolyte, the material requires significant thermal energy. The 1100°C threshold is generally where the resistance drops low enough to allow the process to be energetically viable.
Enabling Oxygen Mobility
The process relies on "pumping" oxygen ions out of the titanium melt and through the solid membrane.
At temperatures approaching 1300°C, the crystal lattice of the membrane creates vacancies that allow oxygen ions to hop from one site to another. This high ionic mobility is the engine of the deoxygenation process.
Maintaining the Electrolytic Environment
Ensuring Molten Salt Stability
The secondary requirement for this temperature range is the physical state of the flux. The molten salt system acts as the transfer medium between the titanium cathode and the SOM anode.
The furnace must maintain a temperature well above the melting point of these salts. This ensures low viscosity, which promotes better mass transfer and prevents the solidification of the flux near cooler zones of the reactor.
Understanding the Trade-offs
The Balance of Efficiency vs. Stability
Operating at the higher end of the spectrum (1300°C) maximizes ionic conductivity, making the process faster and more electrically efficient.
However, extreme temperatures place immense stress on furnace components.
Material Limitations
While higher temperatures improve reaction kinetics, they also accelerate the degradation of the furnace lining and the membrane itself.
Furthermore, as noted in general high-temperature processing, specialized furnace technologies (such as those used in sintering at similar ranges) are required to maintain atmospheric control and temperature uniformity at these extremes.
Making the Right Choice for Your Experiment
To determine where in the 1100°C–1300°C range you should operate, consider your specific constraints:
- If your primary focus is process speed: Aim for the upper end (1250°C–1300°C) to maximize the ionic conductivity of the zirconia membrane and reduce reaction time.
- If your primary focus is equipment longevity: Operate closer to the lower bound (1100°C–1150°C) to minimize thermal shock and extend the lifespan of the membrane and heating elements.
Ultimately, the optimal temperature is the point where ionic resistance is minimized without compromising the structural integrity of the SOM apparatus.
Summary Table:
| Factor | 1100°C (Lower Bound) | 1300°C (Upper Bound) |
|---|---|---|
| Ionic Conductivity | Threshold activation; higher resistance | Maximum mobility; peak efficiency |
| Process Speed | Slower deoxygenation rates | Rapid electrochemical reaction |
| Equipment Life | Reduced thermal stress; longer lifespan | Accelerated membrane/lining degradation |
| Physical State | Stable molten salt electrolyte | Low-viscosity salt for fast mass transfer |
Maximize Your Experimental Precision with KINTEK
Achieving the perfect balance between ionic mobility and equipment longevity requires superior thermal control. KINTEK provides high-performance laboratory solutions tailored for advanced metallurgy and materials science. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet the rigorous 1100°C–1300°C demands of SOM titanium processing.
Ready to elevate your research efficiency? Contact our technical experts today to find the ideal high-temperature furnace for your unique needs.
Visual Guide
References
- Yuhang Miao, Jinming Hu. Research Progress of Preparing Titanium Alloy By Molten Salt Method. DOI: 10.62051/ijnres.v2n1.30
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the typical lifespan of a vacuum furnace chiller? Extend It to 15+ Years with Proper Care
- What types of materials or products are vacuum annealing furnaces primarily used for? Essential for High-Value, Reactive Materials
- Why is a vacuum sintering furnace essential for Ti-5Al-4W-2Fe alloys? Prevent Embrittlement & Maximize Density
- What material limitations do vacuum furnaces have? Avoid Contamination and Ensure Process Purity
- How is temperature controlled in a vacuum furnace? Achieve Precise Heat Treatment for Your Materials
- What is the significance of using a vacuum drying oven for silicon electrode slurries? Achieve Robust Battery Integrity
- Why is a vacuum drying oven required for Na2O pretreatment? Ensure Pure Molten Oxide Electrolysis Results
- What features ensure the vacuum sintering furnace meets fast heating process requirements? Key Components for Rapid Thermal Cycling



















