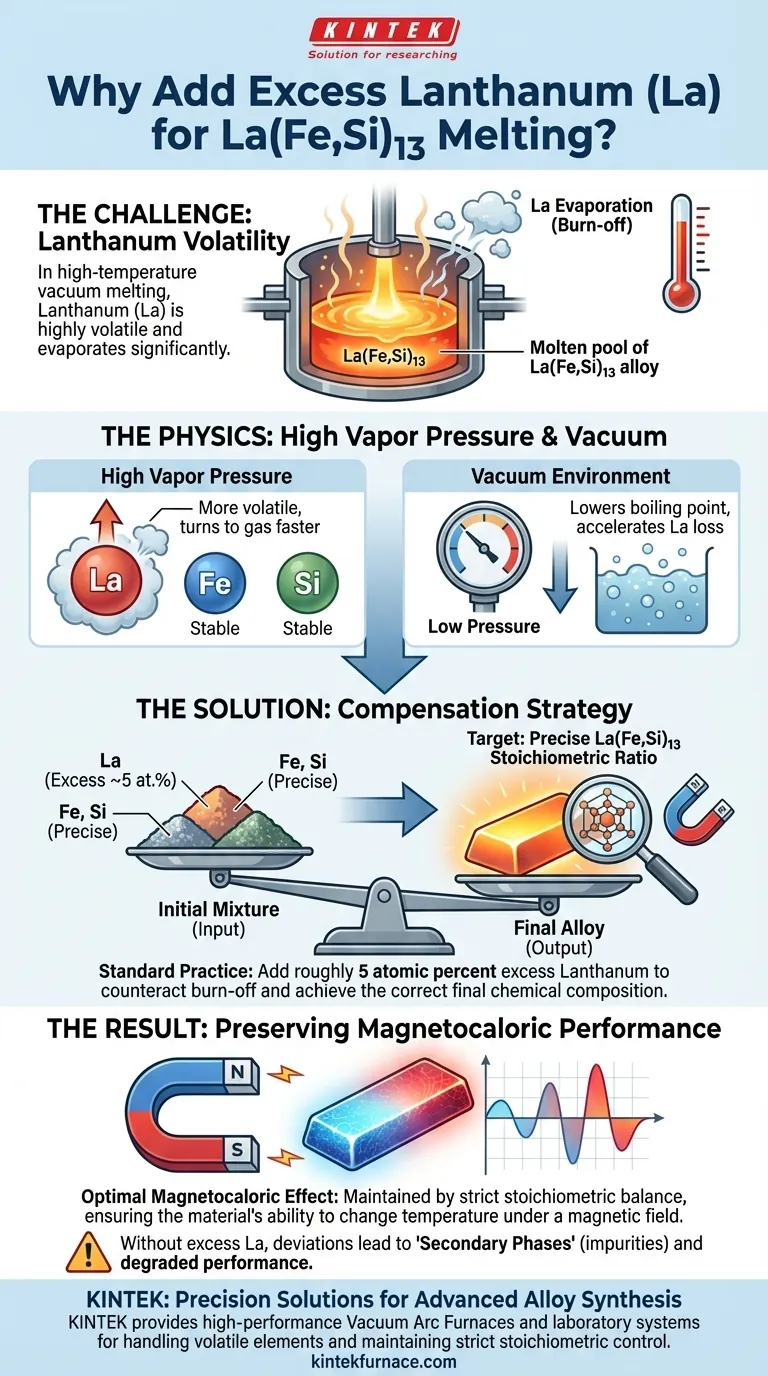
The addition of excess Lanthanum is a critical compensation strategy. In the high-temperature environment of a vacuum arc furnace, Lanthanum (La) is highly volatile and evaporates rapidly. To offset this inevitable loss and ensure the final alloy retains the correct chemical composition, you must introduce a calculated surplus of the metal at the start of the process.
Core Insight: High-temperature vacuum melting causes significant "burn-off" of volatile elements due to high vapor pressure. Adding a precise surplus of Lanthanum (typically 5 atomic percent) counteracts this evaporation, preserving the strict stoichiometric ratio required for optimal magnetocaloric performance.

The Physics of Vacuum Arc Melting
High Vapor Pressure
During the melting process, the alloy is subjected to intense heat. Lanthanum exhibits high vapor pressure relative to other components in the La(Fe,Si)13 mixture.
This physical property makes the metal unstable in liquid form at high temperatures. It tends to transition into a gas much faster than the iron or silicon components.
The Phenomenon of Burn-off
The vacuum environment further accelerates this instability. As the pressure in the chamber drops, the boiling point of the metal decreases, leading to significant evaporative loss.
This loss is technically referred to as "burn-off." Without intervention, this phenomenon would leave the final alloy deficient in Lanthanum.
Achieving Stoichiometric Precision
Compensating with Excess Material
To counteract burn-off, you cannot simply weigh out the exact theoretical ratio of the alloy. You must add an excess amount of Lanthanum to the initial mixture.
Standard practice typically dictates an addition of roughly 5 atomic percent above the target formula. This surplus is sacrificial; it is intended to be lost during the melt so that the remaining material hits the target.
Preserving Magnetocaloric Performance
The ultimate goal of this compensation is to maintain the precise stoichiometric ratio of the La(Fe,Si)13 phase.
The magnetic properties of these alloys are extremely sensitive to their chemical balance. If the Lanthanum content drops below the required ratio, the magnetocaloric effect—the material's ability to change temperature under a magnetic field—will be compromised.
Managing Compositional Risks
The Consequence of Imbalance
While adding excess is necessary, it introduces a challenge in process control. The goal is to balance the input surplus exactly against the output loss.
Inconsistent Evaporation Rates
If the vacuum pressure or arc temperature fluctuates, the rate of evaporation may change. This can lead to a final product that is either still distinctively Lanthanum-poor or inadvertently Lanthanum-rich.
Formation of Secondary Phases
Failure to hit the strict stoichiometric target does not just weaken the alloy; it can prevent the correct crystal structure from forming entirely. This results in secondary phases that act as impurities, diluting the material's efficiency.
Ensuring Alloy Quality
To maximize the performance of La(Fe,Si)13-based alloys, you must view the initial mixture as a dynamic variable rather than a static recipe.
- If your primary focus is Compositional Accuracy: Ensure your initial weight calculations include the standard 5 atomic percent Lanthanum surplus to offset burn-off.
- If your primary focus is Magnetocaloric Performance: Prioritize the maintenance of the strict stoichiometric ratio, as deviations will directly degrade the alloy's thermal response.
Strict control over initial composition is the only way to guarantee the integrity of the final magnetic material.
Summary Table:
| Factor | Impact on La(Fe,Si)13 Alloys | Mitigation Strategy |
|---|---|---|
| Vapor Pressure | High volatility leads to rapid evaporation (burn-off) | Add ~5 at.% excess Lanthanum |
| Vacuum Environment | Lowers boiling point, accelerating metal loss | Precise pressure/temperature control |
| Stoichiometry | Deviations degrade magnetocaloric properties | Ensure final ratio hits the 1:13 phase |
| Secondary Phases | Compositional imbalance creates unwanted impurities | Strict initial weight calculation |
Optimize Your Advanced Material Synthesis with KINTEK
Precision is paramount when melting sensitive alloys like La(Fe,Si)13. KINTEK provides high-performance laboratory solutions, including Vacuum Arc Furnaces, Muffle, Tube, and CVD systems, specifically engineered to handle volatile elements and maintain strict stoichiometric control. Backed by expert R&D and manufacturing, our equipment is fully customizable to meet your unique research or production requirements.
Ready to achieve superior alloy purity? Contact KINTEK today to consult with our experts and find the perfect high-temperature furnace for your lab.
Visual Guide
References
- Fengqi Zhang, Yang Ren. Engineering Light‐Element Modified LaFe <sub>11.6</sub> Si <sub>1.4</sub> Compounds Enables Tunable Giant Magnetocaloric Effect. DOI: 10.1002/advs.202416288
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Induction Melting Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- Why is a vacuum electric arc furnace essential for Ti-Al alloys? Achieve Superior Metal Purity & Homogeneity
- What is vacuum induction melting technology and why is it important? Achieve High-Purity Metals for Critical Applications
- What are the cost implications of using vacuum or protective atmosphere induction furnaces? Invest in Purity for High-Value Materials
- What role does the induction coil play in an induction melting furnace? It's the Engine of Efficient Melting
- What is the purpose of maintaining high-purity argon gas in vacuum induction melting? Stabilize Your Steel Composition
- What is the objective of using a high-power induction heating system? Optimize High-Entropy Alloy Melting
- What makes induction vacuum melting possible? Unlock Ultra-Pure Metal Production
- How does electromagnetic stirring in IGBT induction melting furnaces improve melt quality? Achieve Superior Metal Purity and Homogeneity



















