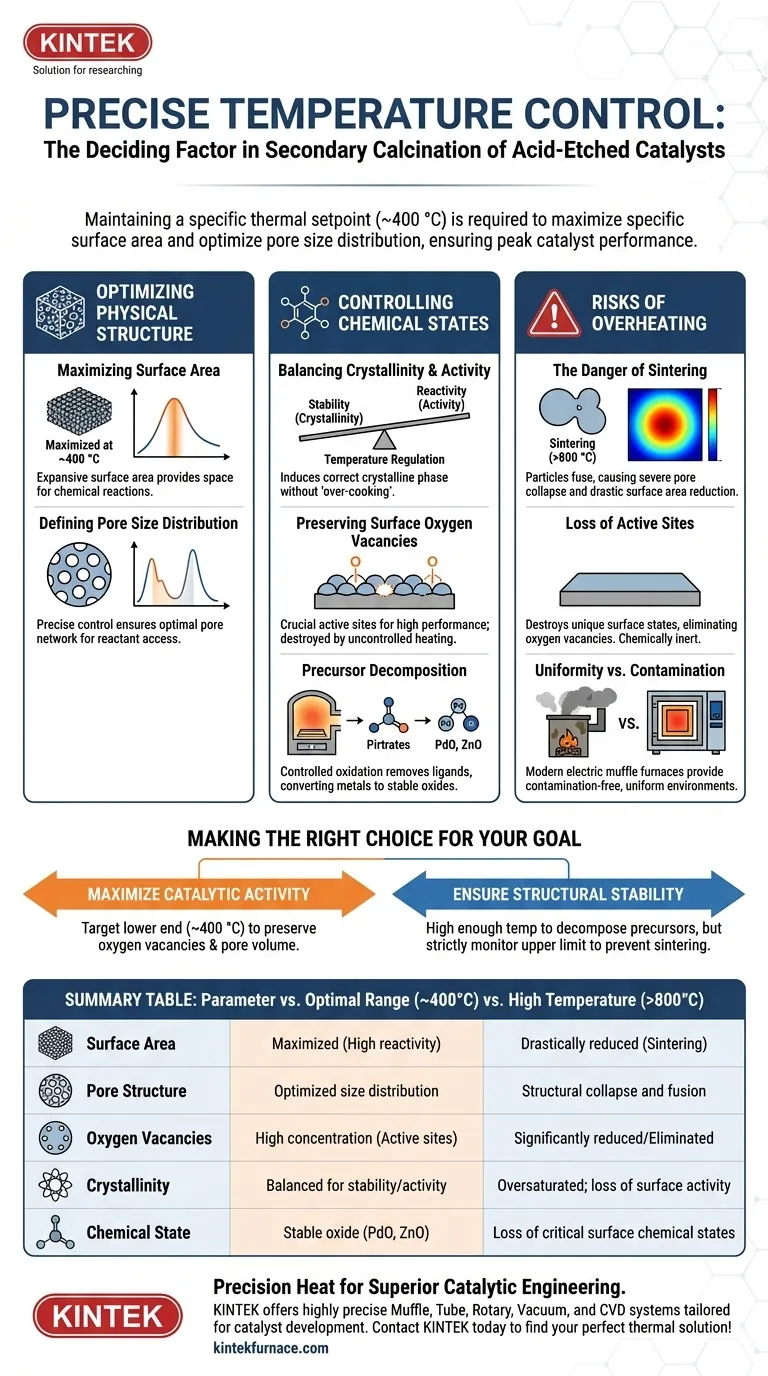
Precise temperature control is the deciding factor in determining whether an acid-etched catalyst achieves peak performance or suffers irreversible structural failure. During secondary calcination, maintaining a specific thermal setpoint—typically around 400 °C—is required to maximize specific surface area and optimize pore size distribution. Without this precision, the process risks failing to balance the material's crystallinity with its necessary surface activity.
Accurate regulation of the muffle furnace enables the delicate trade-off between hardening the material structure and preserving active surface sites. It ensures the catalyst retains high concentrations of surface oxygen vacancies while preventing the structural collapse associated with overheating.

Optimizing Physical Structure
Maximizing Surface Area
The primary goal of secondary calcination is to solidify the physical framework of the catalyst. Research indicates that calcining at an optimal temperature, such as 400 °C, achieves the maximum specific surface area.
This expansive surface area is critical because it provides the physical space necessary for chemical reactions to occur.
Defining Pore Size Distribution
Beyond just surface area, the quality of the surface matters. Precise heat control ensures an optimal pore size distribution within the material.
If the temperature fluctuates or drifts, the pore network can become irregular, potentially blocking reactants from reaching active sites.
Controlling Chemical States
Balancing Crystallinity and Activity
Temperature regulation acts as a lever to balance two competing needs: material crystallinity (stability) and surface activity (reactivity).
The muffle furnace must provide enough energy to induce the correct crystalline phase without "over-cooking" the material. This balance directly impacts the catalyst's longevity and efficiency.
Preserving Surface Oxygen Vacancies
For acid-etched catalysts, surface oxygen vacancies are often the key to high performance. These vacancies act as active sites for many catalytic processes.
Strict temperature limits are required to preserve these vacancies; uncontrolled heating can anneal the surface too smoothly, eliminating these critical imperfections.
Precursor Decomposition
The furnace must also provide a controlled oxidation environment to remove ligands such as nitrates or acetylacetonates.
By maintaining constant temperature stages, the furnace ensures these precursors decompose completely, converting metal components into stable oxide states like palladium oxide or zinc oxide.
Understanding the Trade-offs: The Risks of Overheating
The Danger of Sintering
The most significant risk in this process is sintering, which occurs when temperatures exceed the optimal range (e.g., reaching 800 °C).
Sintering causes the catalyst particles to fuse together. This leads to a severe collapse of the pore structure and a drastic reduction in surface area.
Loss of Active Sites
When sintering occurs, the unique surface chemical states created by acid etching are destroyed.
Specifically, high temperatures reduce the concentration of surface oxygen vacancies. The result is a mechanically stable but chemically inert material that fails to function as a catalyst.
Uniformity vs. Contamination
While older combustion-based furnaces could achieve high heat, they introduced combustion byproducts that could contaminate sensitive samples.
Modern electric muffle furnaces eliminate this trade-off. They provide a contamination-free environment with high uniformity, ensuring that the "sintering threshold" is not accidentally crossed in localized hot spots.
Making the Right Choice for Your Goal
To ensure the success of your secondary calcination process, you must align your thermal strategy with your material's specific limitations.
- If your primary focus is maximizing catalytic activity: Target the lower end of the effective calcination range (around 400 °C) to preserve the highest density of surface oxygen vacancies and pore volume.
- If your primary focus is structural stability: Ensure the temperature is high enough to fully decompose precursors and ligands, but strictly monitor the upper limit to prevent the onset of sintering.
Ultimately, the muffle furnace should be viewed not merely as a heating device, but as a precision instrument for engineering the microscopic architecture of your catalyst.
Summary Table:
| Parameter | Optimal Range (~400°C) | High Temperature (>800°C) |
|---|---|---|
| Surface Area | Maximized for high reactivity | Drastically reduced (Sintering) |
| Pore Structure | Optimized size distribution | Structural collapse and fusion |
| Oxygen Vacancies | High concentration (Active sites) | Significantly reduced/Eliminated |
| Crystallinity | Balanced for stability/activity | Oversaturated; loss of surface activity |
| Chemical State | Stable oxide (PdO, ZnO) | Loss of critical surface chemical states |
Precision Heat for Superior Catalytic Engineering
Don't let sintering compromise your research. Backed by expert R&D and world-class manufacturing, KINTEK offers highly precise Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for the delicate needs of catalyst development. Our lab high-temp furnaces are fully customizable to ensure uniform heating and contamination-free environments, preserving the critical oxygen vacancies and pore structures your acid-etched catalysts require.
Ready to achieve peak performance? Contact KINTEK today to find the perfect thermal solution for your lab!
Visual Guide
References
- Li Yang, Zongping Shao. Rational Design of a Perovskite‐Type Catalyst for Toluene Oxidation Via Simultaneous Phosphorus Doping and Post‐Synthesis Acidic Etching. DOI: 10.1002/eem2.70115
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What role does a high-temperature muffle furnace play in determining ash content? Expert Inorganic Analysis Guide
- Why are muffle furnaces not suitable for low-temperature applications? Discover the High-Temperature Design Limits
- How are muffle furnaces used in forensic investigations? Uncover Hidden Evidence with Precision Ashing
- What industries commonly use electric muffle furnaces? Essential for Precise High-Temp Processing
- What is the function of a Muffle Furnace in date stone carbonization? Optimize Your Bio-Activated Carbon Production
- Why is a high-temp muffle furnace required for graphene catalyst calcination? Achieve Precise Phase Transformation
- How is a laboratory muffle furnace used in 3D-printed PP-CF cross-linking? Achieve Thermal Stability at 150 °C
- What role does a high-temperature box resistance furnace play in Hydroxyapatite/Zirconia composite preparation?



















