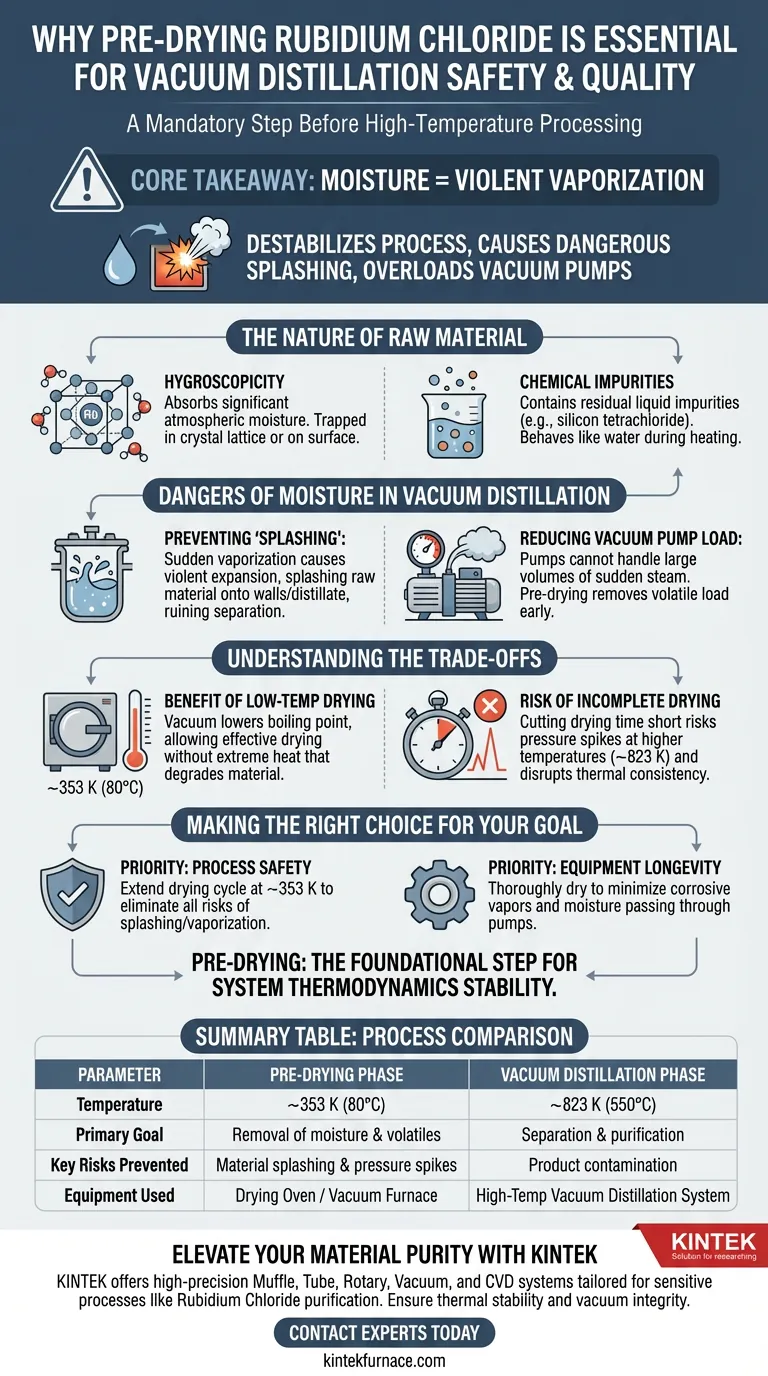
Pre-drying Rubidium Chloride is a mandatory safety and quality assurance step required because the material is highly hygroscopic and often retains volatile impurities. By heating the raw materials in a drying oven or vacuum furnace at 353 K, you remove absorbed water, crystal water, and liquid residues like silicon tetrachloride before they can disrupt the sensitive vacuum distillation process.
Core Takeaway Introducing moisture into a high-temperature vacuum system causes violent vaporization that destabilizes the entire process. Pre-drying ensures a stable chemical baseline, prevents dangerous material splashing, and protects the vacuum pump system from excessive load.

The Nature of the Raw Material
Addressing Hygroscopicity
Rubidium Chloride is highly hygroscopic, meaning it naturally absorbs significant moisture from the surrounding atmosphere.
If this water is not removed prior to processing, it remains trapped within the crystal lattice or on the surface of the material.
Removing Chemical Impurities
Beyond simple water, the raw material often contains residual liquid impurities, such as silicon tetrachloride.
These residues behave similarly to water during heating and must be evacuated to ensure the purity of the final product.
Why Moisture is Dangerous in Vacuum Distillation
Preventing "Splashing"
The most critical reason for pre-drying is to prevent splashing caused by sudden vaporization.
When water enters a high-temperature vacuum environment, it turns to steam almost instantly and expands violently.
This rapid expansion creates physical turbulence that can splash raw material onto the reactor walls or into the distillate, ruining the separation process.
Reducing Vacuum Pump Load
Vacuum pumps are designed to maintain low pressure, not to evacuate large volumes of suddenly generated steam.
Pre-drying removes the volatile load early, ensuring the vacuum system can maintain stable pressure during the subsequent, critical distillation phase.
Understanding the Trade-offs
The Benefit of Low-Temperature Drying
Using a vacuum furnace allows you to dry the material effectively at lower temperatures (around 353 K) compared to atmospheric drying.
The vacuum environment lowers the boiling point of water and solvents, allowing them to evaporate without requiring extreme heat that might prematurely degrade the raw material.
The Risk of Incomplete Drying
If you cut the drying time short to save time, you risk pressure spikes later in the process.
Even small amounts of residual moisture can disrupt the thermal consistency required for distillation, which typically occurs at much higher temperatures (approx. 823 K).
Making the Right Choice for Your Goal
To optimize your distillation process, prioritize your drying parameters based on your specific operational constraints:
- If your primary focus is Process Safety: Ensure the drying cycle at 353 K is extended long enough to eliminate all risks of splashing or sudden vaporization.
- If your primary focus is Equipment Longevity: Thoroughly dry materials to minimize the volume of corrosive vapors and moisture passing through your vacuum pumps.
Pre-drying is not merely a suggestion; it is the foundational step that stabilizes the thermodynamics of your entire vacuum system.
Summary Table:
| Parameter | Pre-Drying Phase | Vacuum Distillation Phase |
|---|---|---|
| Temperature | ~353 K (80°C) | ~823 K (550°C) |
| Primary Goal | Removal of moisture & volatiles | Separation & purification |
| Key Risks Prevented | Material splashing & pressure spikes | Product contamination |
| Equipment Used | Drying Oven / Vacuum Furnace | High-Temp Vacuum Distillation System |
Elevate Your Material Purity with KINTEK
Don't let moisture compromise your vacuum distillation results. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for sensitive chemical processes like Rubidium Chloride purification. Whether you need a standard lab furnace or a fully customizable high-temperature system, our equipment ensures the thermal stability and vacuum integrity your research demands.
Ready to optimize your lab's efficiency and process safety?
Contact our technical experts today to find your perfect solution!
Visual Guide
References
- Cui Xi, Tao Qu. A Study on the Removal of Impurity Elements Silicon and Zinc from Rubidium Chloride by Vacuum Distillation. DOI: 10.3390/ma17091960
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the primary purpose of using a vacuum drying oven to treat master alloy powders? Ensure Purity & Prevent Oxidation
- What are the technical advantages of using an Aerodynamic Levitation Furnace? Achieve Ultra-Pure Silicate Melt Research
- What are the advantages of using a vacuum drying oven for BiOCl precursors? Ensure Purity and Sintering Efficiency
- Why is vacuum degassing equipment utilized in the preparation of low-alloy fire-resistant steel? | KINTEK Solutions
- Why is a laboratory vacuum drying oven necessary for sodium-ion battery half-cells? Achieve Peak Battery Performance
- Why has vacuum heat treatment technology gained widespread use? Achieve Superior Material Control and Performance
- How do multiple-chamber vacuum furnaces enhance productivity? Boost Throughput with Continuous Workflow
- Why is a vacuum environment essential when using a Spark Plasma Sintering (SPS) furnace for Ti64-Si3N4-ZrO2? Achieve Optimal Density & Purity



















