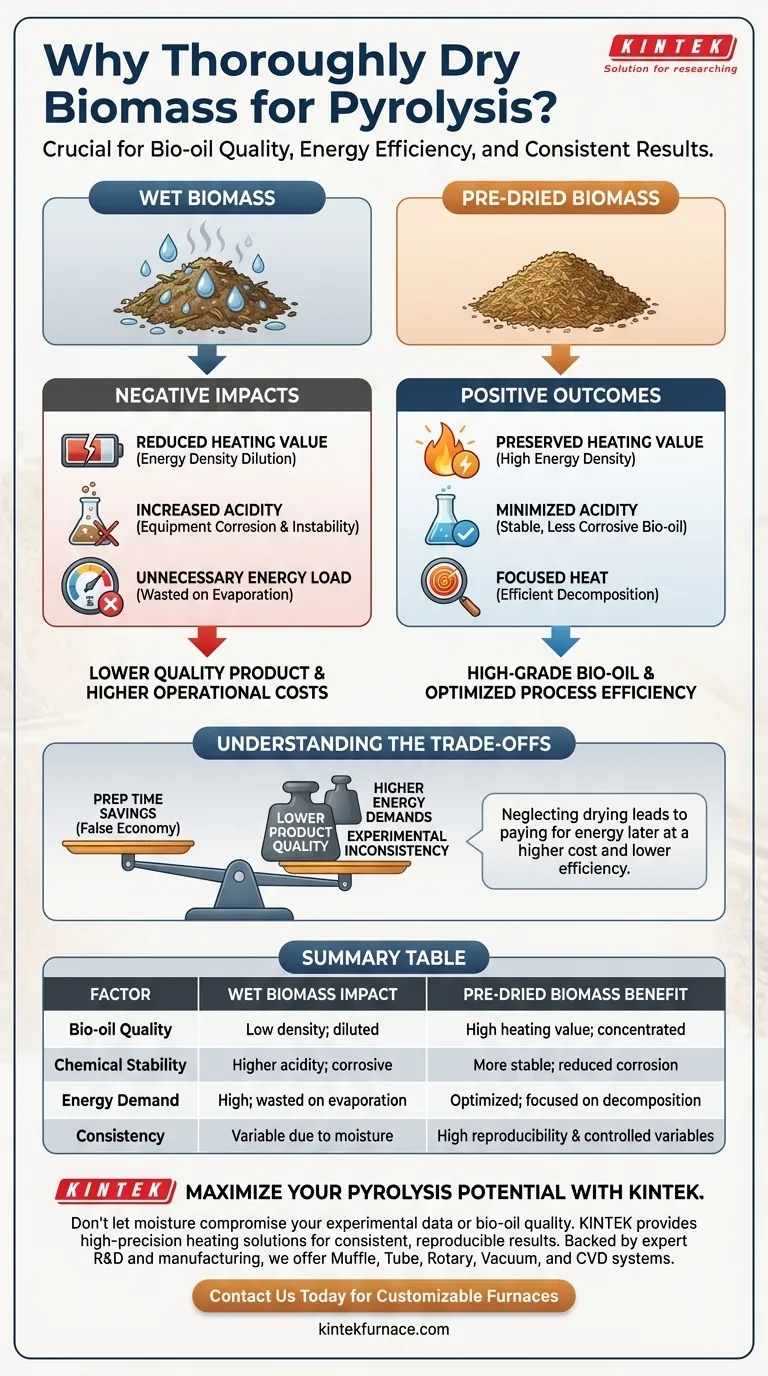
Thoroughly drying biomass ensures the removal of physical moisture, which is a critical prerequisite for a successful pyrolysis experiment. By lowering the feedstock's moisture content before the process begins, you directly enhance the quality of the resulting bio-oil and significantly reduce the thermal energy required to run the reactor.
Moisture acts as a parasitic load on the pyrolysis process. Eliminating it beforehand preserves the heating value of your final bio-oil, minimizes corrosive acidity, and ensures that your energy input is directed toward chemical conversion rather than water evaporation.

Enhancing the Quality of the Final Product
To achieve a high-grade bio-oil, the chemical composition must be protected from the dilution effects of water.
Preserving Heating Value
The primary goal of many pyrolysis experiments is to produce a fuel source. Water has no caloric value.
If the raw material retains physical moisture, that water inevitably transfers into the final bio-oil product. This dilution significantly reduces the heating value (energy density) of the fuel, making it less efficient for combustion or further processing.
Minimizing Acidity
High water content does more than just dilute the fuel; it chemically alters the product's stability.
The presence of excess moisture is linked to increased acidity in the final bio-oil. Acidic bio-oil is chemically unstable and can be corrosive to storage containers, engines, and refining equipment.
Optimizing Thermal Energy Consumption
Beyond the product quality, drying is a matter of thermodynamic efficiency.
Eliminating Unnecessary Energy Load
Pyrolysis is an endothermic process that requires heat to break chemical bonds.
If the biomass is wet, the reactor must first expend significant energy simply to heat and evaporate the water. This is unnecessary thermal energy consumption that contributes nothing to the actual pyrolysis reaction.
Focusing Heat on Decomposition
By removing moisture prior to the experiment, you ensure the reactor's energy is applied efficiently.
Heat is directed immediately toward decomposing the biomass material rather than overcoming the latent heat of vaporization of water. This leads to a faster, more controllable, and more energy-efficient experimental run.
Understanding the Trade-offs
While drying is essential, it is important to recognize the consequences of neglecting this step.
The "False Economy" of Skipping Drying
One might attempt to skip drying to save preparation time or pre-processing costs.
However, this creates a trade-off where you essentially pay for that energy later in the reactor, often at a higher cost and with lower efficiency. You trade a small amount of prep time for a lower quality product and higher operational energy demands.
Impact on Experimental Consistency
Moisture content in biomass can vary wildly depending on storage conditions.
If you do not dry the material thoroughly to a known baseline, your experiments will suffer from inconsistent variables. High water content introduces variables that make it difficult to replicate results or isolate the effects of other parameters.
Making the Right Choice for Your Experiment
Proper preparation of your feedstock is the single most effective way to ensure reliable data and usable product.
- If your primary focus is Bio-oil Quality: Thorough drying is mandatory to maximize heating value and prevent the formation of highly acidic, unstable oil.
- If your primary focus is Energy Efficiency: Drying the feedstock externally is usually more efficient than forcing the pyrolysis reactor to boil off excess water.
- If your primary focus is Equipment Longevity: Reducing moisture lowers the acidity of the resulting oil, protecting your downstream equipment from corrosion.
Start with dry material to ensure you are measuring the potential of your biomass, not the limitations of your process.
Summary Table:
| Factor | Wet Biomass Impact | Pre-Dried Biomass Benefit |
|---|---|---|
| Bio-oil Quality | Low energy density; highly diluted | High heating value; concentrated fuel |
| Chemical Stability | Higher acidity; corrosive potential | More stable; reduced equipment corrosion |
| Energy Demand | High (energy wasted on water evaporation) | Optimized (heat focused on decomposition) |
| Consistency | Variable results due to moisture fluctuations | High reproducibility & controlled variables |
Maximize Your Pyrolysis Potential with KINTEK
Don't let moisture compromise your experimental data or bio-oil quality. KINTEK provides high-precision heating solutions designed to deliver consistent, reproducible results. Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as specialized lab high-temp furnaces—all fully customizable to meet your unique biomass processing needs.
Ready to optimize your thermal conversion process? Contact us today to speak with our technical experts and find the perfect furnace for your laboratory.
Visual Guide
References
- Haniif Prasetiawan, R Fitrah. The Effect of Raw Material Composition and Pyrolysis Temperature on The Characteristics of Bio-Oil from the Pyrolysis of Sawdust and Sugar Cane Bagasse Mixture. DOI: 10.1051/e3sconf/202564803007
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the specific temperature control requirements for alpha-SiC growth? Master the 1700°C Thermal Threshold
- What is the significance of the 200 °C calcination for Fe3O4/biochar? Enhancing Stability and Magnetic Recovery
- What is the function of a precise heating system during the hydrolysis of palm kernel oil? Optimize Your Fatty Acid Yield
- What advantages does peat char offer compared to traditional charcoal? Boost Your Furnace Efficiency by 22%
- How are laboratory ovens and analytical balances used for banana powder moisture content? Precision Testing Guide
- Why is a homogeneous reactor used for crystallization? Achieve 100% Thermal Uniformity for Pure Crystals
- Why is a vacuum desiccator used for the preservation of extracted fruit peel extracts? Protect Bioactive Compounds
- What are the advantages of using microwave drying equipment for organic gels? Preserve Pore Structures Effectively



















