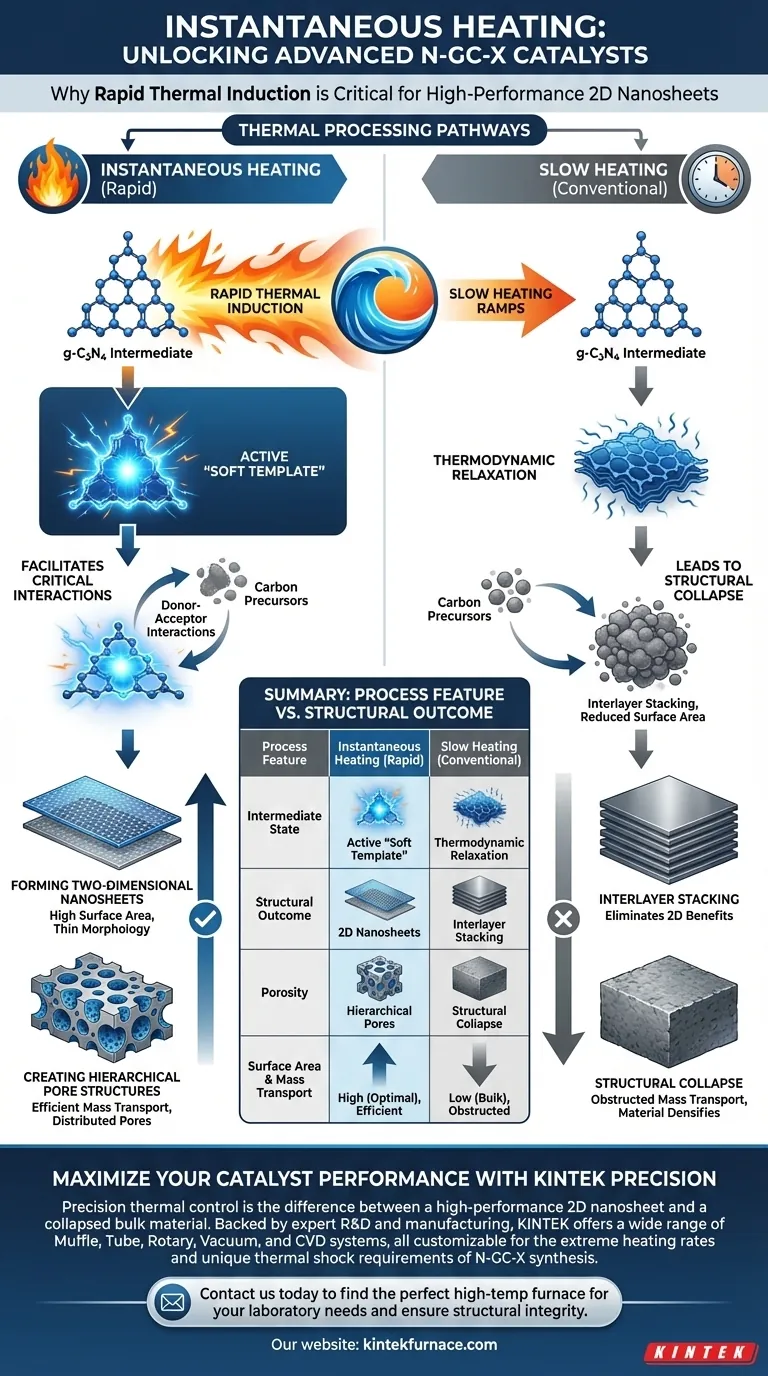
Instantaneous heating is strictly required to generate the extremely high heating rates necessary for transforming the g-C3N4 intermediate into an effective soft template. Without this rapid thermal induction, the essential donor-acceptor interactions with carbon precursors cannot be properly sustained to shape the final catalyst.
By utilizing rapid thermal induction, you prevent the structural collapse inherent to slower heating methods. This specific thermal shock is the only way to preserve the interactions required to form two-dimensional carbon nanosheets with hierarchical porosity.

The Mechanism of Soft Templating
Activating the Intermediate
The primary function of instantaneous heating is to activate the g-C3N4 intermediate.
Under high heating rates, this compound behaves as a "soft template." This state is transient and requires rapid energy input to be maintained effectively during synthesis.
Facilitating Donor-Acceptor Interactions
Once active as a soft template, g-C3N4 engages in critical donor-acceptor interactions with the carbon precursors.
These chemical interactions guide the assembly of the material. They are responsible for directing the carbon precursors into the desired architectural configuration rather than a random bulk mass.
Structural Implications
Forming Two-Dimensional Nanosheets
The ultimate goal of this synthesis is the creation of two-dimensional carbon nanosheets.
The rapid thermal induction allows these thin, sheet-like structures to form. This morphology offers a significant surface area advantage compared to bulk materials.
Creating Hierarchical Pore Structures
Beyond the 2D shape, the catalyst requires a specific internal architecture known as hierarchical porosity.
Instantaneous heating ensures that pores of various sizes are distributed throughout the nanosheets. This structure is vital for mass transport within the catalyst during its final application.
Understanding the Trade-offs
The Risks of Slow Heating
It is critical to understand why standard, slower heating ramps fail in this specific synthesis.
Slow heating allows time for thermodynamic relaxation, which leads to structural collapse. Instead of retaining an open, porous network, the material densifies.
Preventing Interlayer Stacking
A major pitfall of insufficient heating rates is interlayer stacking.
Without the shock of instantaneous heating, the developing carbon layers tend to stack on top of one another. This reduces the exposed surface area and eliminates the benefits of the 2D nanosheet morphology.
Making the Right Choice for Your Synthesis
To ensure you achieve the desired N-GC-X catalyst properties, align your thermal processing with your structural goals:
- If your primary focus is high surface area: You must use instantaneous heating to prevent interlayer stacking and ensure the formation of separated nanosheets.
- If your primary focus is mass transport efficiency: You must prioritize the high heating rate to secure the hierarchical pore structure that prevents structural collapse.
The success of N-GC-X synthesis relies entirely on the speed of thermal induction to lock in the template's structure before it can degrade.
Summary Table:
| Process Feature | Instantaneous Heating (Rapid) | Slow Heating (Conventional) |
|---|---|---|
| Intermediate State | Active "Soft Template" | Thermodynamic Relaxation |
| Structural Outcome | 2D Nanosheets | Interlayer Stacking |
| Porosity | Hierarchical Pores | Structural Collapse |
| Surface Area | High (Optimal) | Low (Bulk Material) |
| Mass Transport | Efficient | Obstructed |
Maximize Your Catalyst Performance with KINTEK Precision
Precision thermal control is the difference between a high-performance 2D nanosheet and a collapsed bulk material. Backed by expert R&D and manufacturing, KINTEK offers a wide range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for the extreme heating rates and unique thermal shock requirements of N-GC-X synthesis.
Don't let slow heating compromise your research. Contact us today to find the perfect high-temp furnace for your laboratory needs and ensure the structural integrity of your advanced materials.
Visual Guide
References
- Ganchang Lei, Lilong Jiang. Atom-economical insertion of hydrogen and sulfur into carbon–nitrogen triple bonds using H<sub>2</sub>S <i>via</i> synergistic C–N sites. DOI: 10.1039/d5ey00110b
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does an industrial fast firing furnace play in the metallization of PERT solar cells? Boost Cell Efficiency
- Why is a precision constant temperature drying oven required for the impregnation modification process of activated carbon?
- What role does high-vacuum thermal evaporation equipment play in CsPbBr3 detectors? Optimize Electrode Fabrication
- How does plant metal-ion absorption influence pyrolysis? Enhance Material Synthesis with Biological Pretreatment
- What role does a vertical heating furnace play in the production of nano-glass composites? Precision Shaping Experts
- What role do high-temp furnaces play in co-firing SOFCs? Master Ceramic Densification and Sintering
- What are the primary advantages of using powder metallurgy for Ti and TiZr alloys? Achieve Ultimate Structural Precision
- What is the necessity of in-situ DRIFTS in formaldehyde oxidation? Uncover Real-Time Catalytic Reaction Mechanisms



















