The necessity of in-situ DRIFTS paired with a temperature control unit lies in its ability to provide direct spectroscopic evidence of the reaction mechanism. By stabilizing specific thermal environments, this setup captures and identifies reaction intermediates on the catalyst surface that would otherwise be invisible to post-mortem analysis.
The combination of in-situ DRIFTS and precise temperature control is the only way to dynamically analyze peak intensities of adsorbed species, proving how the catalyst interface reduces energy barriers via the Langmuir-Hinshelwood mechanism.
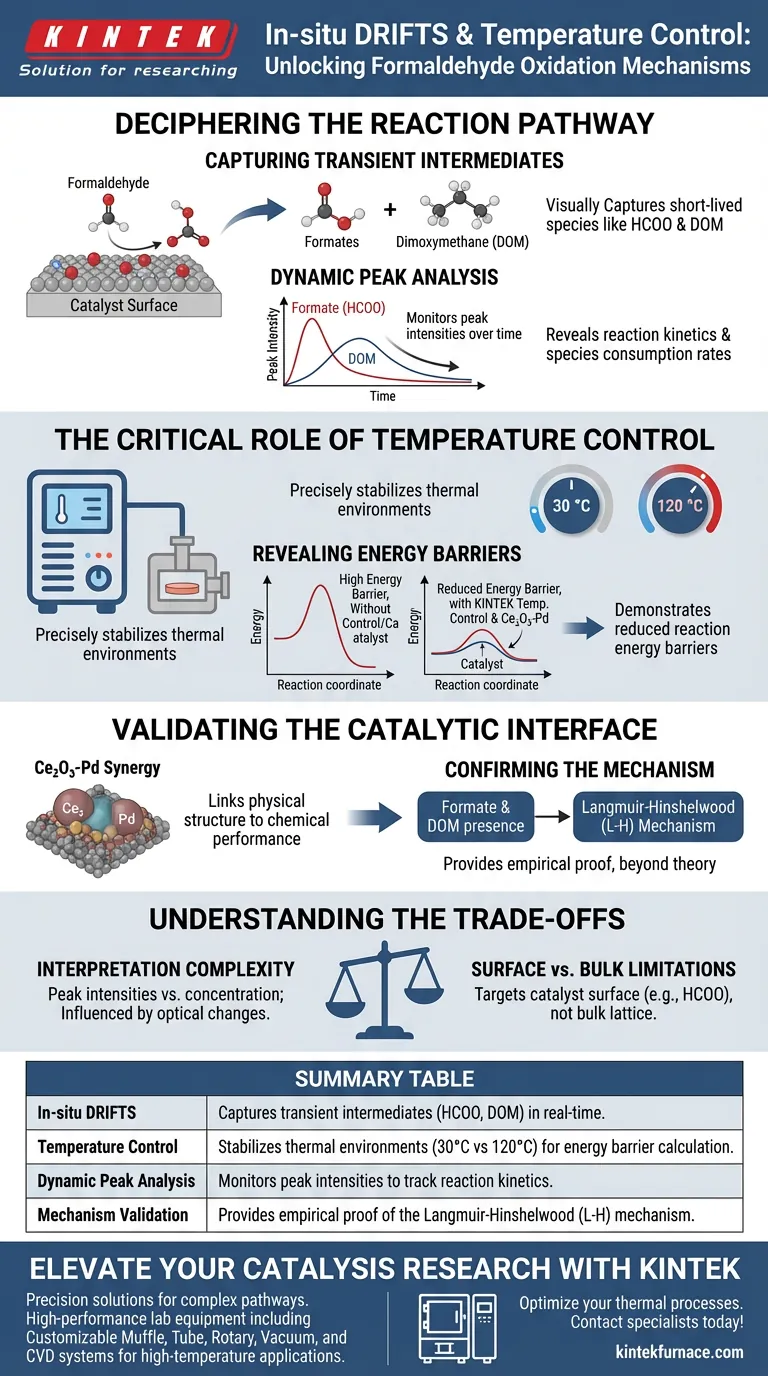
Deciphering the Reaction Pathway
To understand formaldehyde oxidation, you cannot simply look at reactants and products. You must observe the "middle steps" of the chemical journey.
Capturing Transient Intermediates
In-situ DRIFTS allows you to "see" chemical species that exist only briefly on the catalyst surface.
Specifically, it enables the identification of formates (HCOO) and dimoxymethane (DOM). These species are the smoking gun evidence of how the reaction proceeds.
Dynamic Peak Analysis
Static snapshots are insufficient for understanding oxidation mechanisms.
By performing dynamic analysis, researchers monitor the peak intensities of these adsorbed species over time. This data reveals the rate at which intermediates are formed and consumed, providing a clear picture of reaction kinetics.
The Critical Role of Temperature Control
The temperature control unit is not merely an accessory; it is the variable that allows for the calculation of energy barriers.
Precise Thermal Targeting
The system enables the capture of data at specific, relevant operating temperatures, such as 30 °C or 120 °C.
Holding the catalyst at these exact temperatures allows researchers to isolate how heat affects surface adsorption.
revealing Energy Barriers
By comparing spectroscopic data across these temperature points, the system reveals the energy requirements of the reaction.
This analysis demonstrates how the catalyst significantly reduces reaction energy barriers, making the oxidation process more efficient.
Validating the Catalytic Interface
The ultimate goal of using this equipment is to link physical structure to chemical performance.
The Ce2O3-Pd Synergy
The data derived from this setup provides the proof needed to understand specific interfaces, such as Ce2O3-Pd.
It confirms that the interaction between these materials is what drives the reaction efficiency.
Confirming the Mechanism
The presence and behavior of the formate and DOM species specifically point to the Langmuir-Hinshelwood (L-H) mechanism.
Without the ability to track these adsorbed species in real-time, confirming this specific mechanism would be theoretical rather than empirical.
Understanding the Trade-offs
While in-situ DRIFTS is powerful, it is important to recognize the complexities inherent in this analysis.
Interpretation Complexity
Data from DRIFTS relies on the interpretation of peak intensities.
Changes in intensity generally correlate to concentration, but they can also be influenced by changes in the optical properties of the catalyst surface during the reaction.
Surface vs. Bulk Limitations
This technique specifically targets the catalyst surface.
It excels at identifying adsorbed species (like HCOO) but does not provide direct information regarding changes within the bulk lattice of the catalyst material itself.
Making the Right Choice for Your Research
To apply this to your own work on formaldehyde oxidation or similar catalytic processes:
- If your primary focus is determining reaction pathways: Use the temperature control unit to stabilize the reaction at low (30 °C) and high (120 °C) points to track the evolution of formate and DOM peaks.
- If your primary focus is catalyst efficiency: Focus on the dynamic analysis of peak intensities to quantify how effectively your specific interface (e.g., Ce2O3-Pd) lowers energy barriers.
Ultimately, this setup transforms the study of catalysis from theoretical modeling into empirical observation of surface chemistry in action.
Summary Table:
| Feature | Benefit in Formaldehyde Oxidation Study |
|---|---|
| In-situ DRIFTS | Captures transient intermediates (HCOO, DOM) on the catalyst surface in real-time. |
| Temperature Control | Stabilizes thermal environments (e.g., 30°C vs 120°C) to calculate energy barriers. |
| Dynamic Peak Analysis | Monitors peak intensities to track reaction kinetics and species consumption rates. |
| Mechanism Validation | Provides empirical proof of the Langmuir-Hinshelwood (L-H) mechanism. |
Elevate Your Catalysis Research with KINTEK
Precision is the key to unlocking complex chemical pathways. Backed by expert R&D and manufacturing, KINTEK offers high-performance lab solutions including customizable Muffle, Tube, Rotary, Vacuum, and CVD systems designed for rigorous high-temperature applications. Whether you are studying surface adsorption or refining catalyst interfaces, our equipment provides the stability and control your research demands.
Ready to optimize your lab's thermal processes?
Contact our specialists today to find your perfect solution!
Visual Guide
References
- Lina Zhang, Haifeng Xiong. Generating active metal/oxide reverse interfaces through coordinated migration of single atoms. DOI: 10.1038/s41467-024-45483-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Ultra High Vacuum CF Flange Stainless Steel Sapphire Glass Observation Sight Window
People Also Ask
- Why is an industrial-grade drying oven necessary for biomass activation? Ensure Structural Integrity & Yield
- What is the primary function of a high-precision drop furnace? Master Flash Smelting Simulation Kinetics
- Why is 600 °C critical for ZIF-8 carbonization? Achieve Optimal Surface Area and Functional Group Retention
- What are some common applications of laboratory furnaces? Unlock Precision in Material Transformation
- What role does a laboratory oven play in W-doped TiO2? Ensure Precursor Stability for High-Purity Nanopowders
- How does high-purity argon gas affect the production of ultrafine magnesium powder in evaporation-condensation methods? Master Particle Size Control
- What role does industrial heating equipment play in the manufacturing process of 55Si2 spring steel during winding?
- What is the specific function of a high-temperature laboratory furnace during the activation of kaolin-based catalysts?

