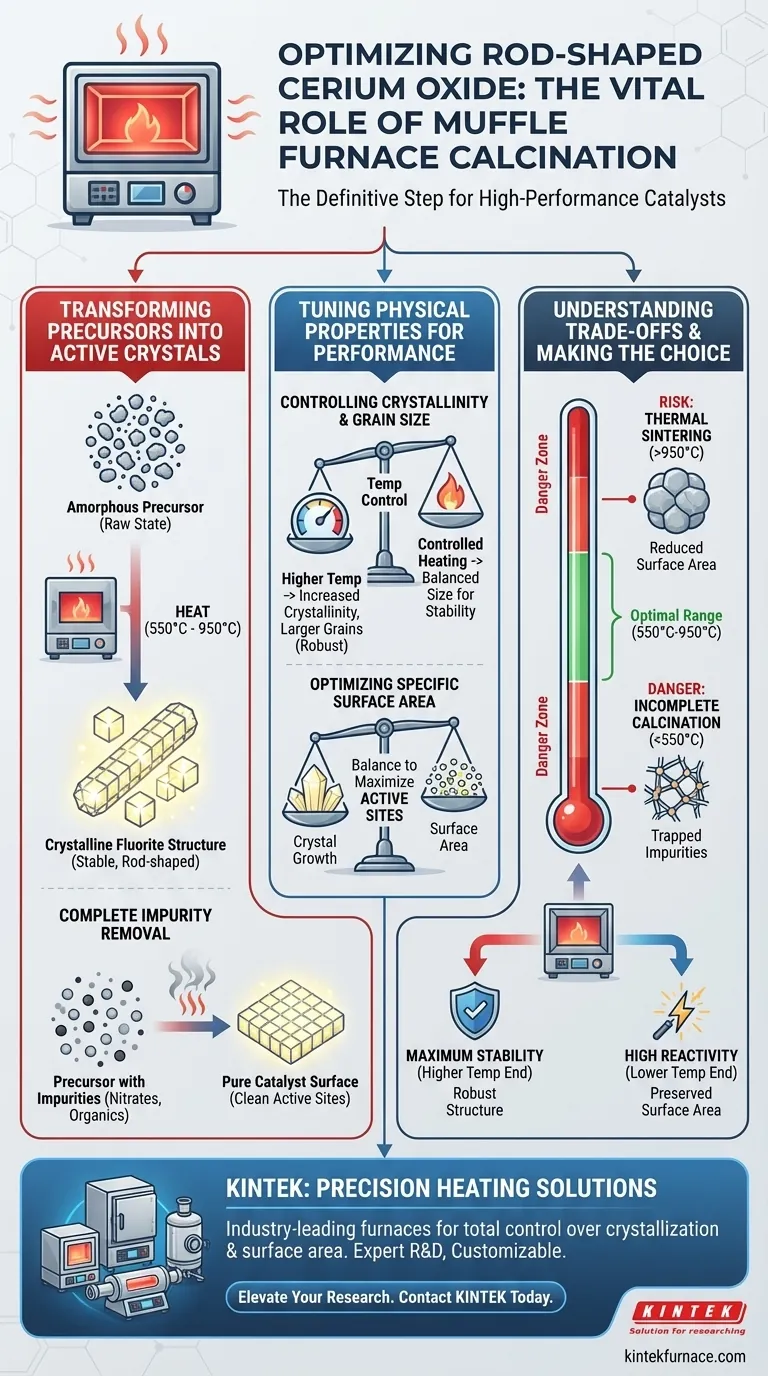
High-temperature calcination is the definitive step that transforms raw chemical precursors into a functional, high-performance catalyst. Specifically, using a muffle furnace allows you to drive the crystallization of cerium oxide into a stable fluorite structure while eliminating residual impurities and optimizing the surface area for maximum reactivity.
By applying a precisely controlled ramped heating process, the muffle furnace facilitates the complete conversion of precursors into pure cerium oxide crystals. This thermal treatment is essential for removing anionic impurities and tuning the grain size to maximize the density of active sites on the catalyst surface.

Transforming Precursors into Active Crystals
Achieving the Stable Fluorite Structure
The primary goal of calcination is phase transformation. You are moving from a raw, often amorphous precursor state to a crystalline oxide.
In a high-temperature muffle furnace, typically operating between 550°C and 950°C, the thermal energy forces the atomic lattice to rearrange. This results in the formation of the stable fluorite crystal structure characteristic of high-performance cerium oxide.
Complete Removal of Impurities
Precursors used in synthesis often leave behind chemical residues that can poison the final catalyst.
The calcination process effectively burns off these residues. Specifically, it targets and removes residual anionic impurities and ligands (such as nitrates or organic compounds) mentioned in broader synthesis contexts. Eliminating these ensures the active sites are not blocked by synthesis byproducts.
Tuning Physical Properties for Performance
Controlling Crystallinity and Grain Size
The performance of a catalyst is dictated by its microstructure. The muffle furnace allows for precise temperature tuning to adjust this structure.
Higher temperatures generally increase crystallinity, making the rod-shaped structures more robust. However, this also affects the grain size. Controlled heating ensures the grains grow large enough to be stable but not so large that they reduce the material's effectiveness.
Optimizing Specific Surface Area
Catalytic activity relies on the availability of active sites.
By carefully selecting the calcination temperature, you directly influence the specific surface area. A properly optimized process balances crystal growth with the preservation of surface area, ensuring the maximum number of active sites remain exposed for chemical reactions.
Understanding the Trade-offs
The Risk of Thermal Sintering
While heat is necessary for crystallization, excessive heat is detrimental.
If the temperature exceeds the optimal range (towards 950°C or higher), you risk sintering. This causes the individual grains to fuse together, drastically reducing the specific surface area and, consequently, the catalytic activity.
The Danger of Incomplete Calcination
Conversely, failing to reach the necessary temperature results in an under-processed material.
If the temperature is too low, the fluorite structure may not fully form, and residual impurities may remain trapped within the lattice. This leads to a catalyst with poor physical stability and unpredictable chemical behavior.
Making the Right Choice for Your Goal
To maximize the performance of your rod-shaped cerium oxide, you must align your heating profile with your specific performance metrics.
- If your primary focus is Maximum Stability: Aim for the higher end of the temperature spectrum to ensure a fully crystallized, robust fluorite structure that resists degradation.
- If your primary focus is High Reactivity: Target the lower effective temperature range (closer to 550°C) to preserve a higher specific surface area and prevent grain coarsening.
Ultimately, the muffle furnace is not just a heating tool; it is a precision instrument for engineering the atomic-level landscape of your catalyst.
Summary Table:
| Process Objective | Temperature Range | Impact on Catalyst Performance |
|---|---|---|
| Phase Transformation | 550°C - 950°C | Converts precursors into stable fluorite crystal structure |
| Impurity Removal | High Temperature | Eliminates nitrates and organic residues to clear active sites |
| Grain Size Control | Controlled Ramping | Balances structural robustness with high surface area |
| Stability vs Reactivity | Target Specific | High Temp (950°C) for stability; Low Temp (550°C) for reactivity |
Precision heating is the key to engineering high-performance catalysts. KINTEK provides industry-leading muffle, tube, and vacuum furnace systems designed to give you total control over crystallization and surface area optimization. Backed by expert R&D and manufacturing, our customizable high-temperature lab furnaces are the perfect partner for your material synthesis needs. Elevate your catalyst research and contact KINTEK today for a tailored thermal solution.
Visual Guide
References
- Mara Arduino, Fabio Alessandro Deorsola. Understanding the Role of Morphology in the Direct Synthesis of Diethyl Carbonate Over Ceria‐Based Catalysts: An In Situ Infrared and High‐Resolution TEM Study. DOI: 10.1002/cctc.202500140
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What is the primary use of a muffle furnace in the assembly of side-heated resistive gas sensors? Expert Annealing Guide
- What role does a muffle furnace play in the conversion of S-1@TiO2? Achieve Precision Calcination of Nanospheres
- What are the key benefits of using a muffle furnace? Achieve Precise, Contaminant-Free High-Temperature Control
- What are the key technological advancements in modern muffle furnaces? Boost Precision and Efficiency in Your Lab
- What is the function of a laboratory muffle furnace in the post-treatment of BiVO4 photocatalytic electrodes?
- What is the range of a muffle furnace? Choosing the Right Temperature for Your Application
- What are the benefits of using a high-temperature sintering furnace at 350°C for PEEK? Maximize Composite Performance
- How are box furnaces used in industrial applications? Versatile Batch Processing for Heat Treating and More



















