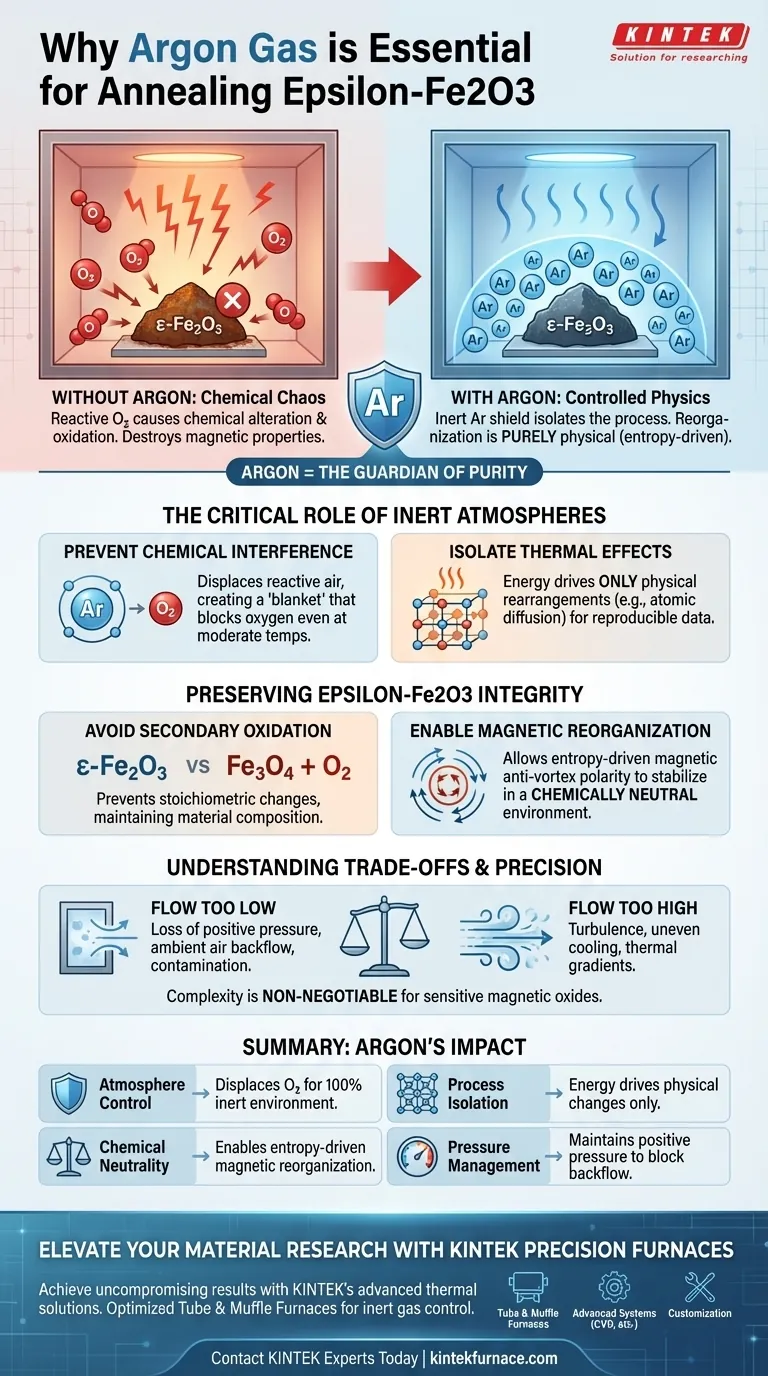
The primary function of an Argon (Ar) gas flow system during the thermal annealing of epsilon-Fe2O3 is to establish and maintain a strictly inert protective atmosphere. This prevents the material from undergoing secondary oxidation or chemical degradation, ensuring that any changes to the structure are purely physical rather than chemical.
The use of Argon isolates the thermal process, ensuring that the reorganization of magnetic properties is driven solely by entropy. Without this inert shield, reactive oxygen in the air would chemically alter the sample, destroying the delicate magnetic characteristics you are trying to study.

The Critical Role of Inert Atmospheres
Preventing Chemical Interference
When materials are heated, their chemical reactivity increases significantly. Even at moderate annealing temperatures (such as 250°C), atmospheric oxygen can react with the surface of a sample.
Argon acts as a barrier. It displaces the air within the muffle or tube furnace, creating a "blanket" that effectively blocks oxygen from reaching the material.
Isolating Thermal Effects
The goal of annealing is often to induce physical changes, such as atomic diffusion or structural relaxation.
By removing chemically reactive gases, you ensure that the energy supplied by the furnace drives only these physical rearrangements. This isolation is critical for obtaining reproducible scientific data.
Preserving Epsilon-Fe2O3 Integrity
Protecting Against Secondary Oxidation
Epsilon-Fe2O3 is a specific phase of iron oxide that is highly sensitive to its environment.
Without an inert gas flow, high temperatures could cause the material to oxidize further or degrade chemically. Secondary oxidation would fundamentally change the stoichiometry of the sample, rendering it useless for specific applications.
Enabling Magnetic Reorganization
The primary reference highlights that this process is intended to affect magnetic properties, specifically the anti-vortex core polarity.
This reorganization is a stochastic (random) process driven by an increase in entropy. For this entropy-driven reorganization to occur correctly, the environment must remain chemically neutral. If the chemical composition changes due to oxidation, the magnetic properties will not stabilize as predicted.
Understanding the Trade-offs
Flow Rate Precision
While Argon provides safety, the flow rate must be carefully managed.
If the flow is too low, positive pressure is lost, and ambient air may backflow into the chamber, contaminating the process. Conversely, if the flow is too high, it can create turbulence or cool the sample surface, leading to uneven heating profiles.
System Complexity and Cost
Using an Argon atmosphere converts a simple heating procedure into a complex system requiring gas tanks, regulators, and sealed furnace chambers (like tube furnaces).
This adds operational cost and setup time compared to air annealing. However, for sensitive semiconductors and magnetic oxides like epsilon-Fe2O3, this complexity is a non-negotiable requirement for success.
Making the Right Choice for Your Goal
When setting up your thermal annealing parameters, consider your specific objective:
- If your primary focus is preserving magnetic fidelity: Ensure a continuous, positive pressure Argon flow to prevent even trace oxidation from altering the anti-vortex core polarity.
- If your primary focus is structural transformation: Monitor the furnace temperature stability closely, as gas flow can occasionally introduce thermal gradients that affect atomic diffusion.
Ultimately, the Argon flow system is the guardian of your sample's purity, converting a chaotic chemical environment into a controlled physics laboratory.
Summary Table:
| Feature | Role of Argon Flow System | Impact on epsilon-Fe2O3 |
|---|---|---|
| Atmosphere Control | Displaces oxygen to create a 100% inert environment. | Prevents secondary oxidation and chemical degradation. |
| Process Isolation | Ensures energy only drives physical atomic rearrangements. | Preserves delicate magnetic anti-vortex core polarity. |
| Chemical Neutrality | Maintains a stable, non-reactive environment. | Enables entropy-driven magnetic reorganization. |
| Pressure Management | Maintains positive pressure to block ambient air backflow. | Ensures reproducible data and stoichiometric purity. |
Elevate Your Material Research with KINTEK Precision Furnaces
Achieve uncompromising results in your epsilon-Fe2O3 research with KINTEK’s advanced thermal solutions. Backed by expert R&D and precision manufacturing, we provide the controlled environments necessary for sensitive magnetic and semiconductor materials.
Our Range of Specialized Solutions Includes:
- Tube & Muffle Furnaces: Optimized for inert gas flow and atmospheric control.
- Advanced Systems: Rotary, Vacuum, and CVD systems for complex processing.
- Customization: Tailored high-temp lab furnaces to meet your unique research specifications.
Don't let atmospheric contamination compromise your magnetic fidelity. Trust KINTEK to provide the stability and purity your experiments demand.
Contact KINTEK Experts Today for a Consultation
Visual Guide
References
- Wuhong Xue, Xiaohong Xu. Stable antivortices in multiferroic ε-Fe2O3 with the coalescence of misaligned grains. DOI: 10.1038/s41467-025-55841-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why is a fixed-bed reactor system with high-precision temperature control necessary for biochar? Achieve +/-3°C Accuracy
- What role does a box resistance furnace play in bamboo carbonization? Master Precision Pyrolysis for Smart Composites
- What role does a high-temperature atmosphere sintering furnace play in BN-Si3N4 sintering? Expert Process Insights
- How does a reducing atmosphere affect heat treatment processes? Enhance Surface Quality and Control
- How do the structural designs and sealing mechanisms differ between box furnaces and atmosphere furnaces? Choose the Right Furnace for Your Lab
- What gases are commonly used in heat treatment furnace atmospheres? Optimize Your Process with the Right Gas Mix
- Why use an air atmosphere furnace for annealing magnesium aluminum spinel? Restoring Lattice & Optical Integrity
- How is a controlled atmosphere furnace used in material research? Achieve Precise Material Synthesis and Heat Treatment



















