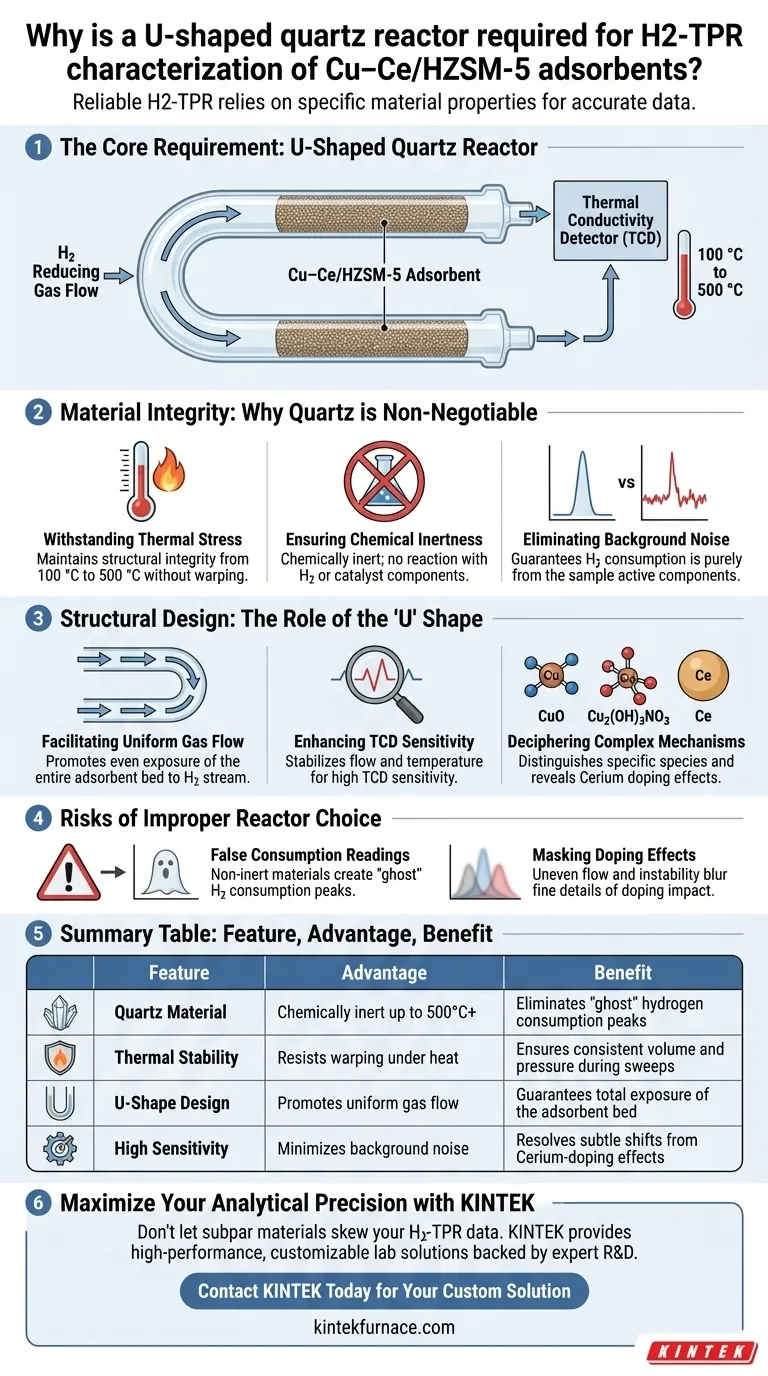
Reliable H2-TPR characterization relies on the specific material properties of the U-shaped quartz reactor. It is required to withstand the critical temperature range of 100 °C to 500 °C while maintaining total chemical inertness. This ensures the reducing gas reacts only with the Cu–Ce/HZSM-5 adsorbent, preventing experimental artifacts from skewing your data.
The U-shaped quartz reactor provides a chemically inert, high-temperature environment that facilitates uniform gas flow. This isolation is critical for accurately detecting subtle reduction peaks associated with cerium-doped copper species using a thermal conductivity detector (TCD).

Material Integrity: Why Quartz is Non-Negotiable
Withstanding Thermal Stress
The reduction process for Cu–Ce/HZSM-5 requires a temperature sweep from 100 °C to 500 °C. Quartz is essential because it maintains structural integrity without warping or softening across this entire thermal gradient.
Ensuring Chemical Inertness
Standard metal reactors can interact with hydrogen or the active components at high temperatures. Quartz is chemically inert, ensuring it does not react with the reducing gas or the catalyst itself.
Eliminating Background Noise
Because the reactor is inert, any hydrogen consumption measured is guaranteed to be from the sample. This purity is vital for attributing data points strictly to the active components of the adsorbent.
Structural Design: The Role of the "U" Shape
Facilitating Uniform Gas Flow
The U-shape geometry is not arbitrary; it promotes a uniform flow of the reducing gas through the adsorbent bed. This ensures every particle of the Cu–Ce/HZSM-5 is exposed to the hydrogen stream equally.
Enhancing TCD Sensitivity
By stabilizing the flow and temperature, the reactor design allows the thermal conductivity detector (TCD) to operate with high sensitivity. This allows for the precise capture of distinct reduction peaks.
Deciphering Complex Mechanisms
This sensitivity is required to distinguish specific chemical species, such as CuO and Cu2(OH)3NO3. Accurately resolving these peaks helps reveal the mechanism by which cerium doping enhances the reduction activity.
Risks of Improper Reactor Choice
False Consumption Readings
Using a reactor material that is not inert can lead to "ghost" hydrogen consumption. This creates false peaks in your data, making it impossible to accurately calculate the reduction degree of the copper species.
Masking Doping Effects
The benefits of cerium doping are often subtle and rely on shifting reduction temperatures or peak shapes. A reactor that fails to maintain uniform flow or thermal stability will blur these fine details, obscuring the actual impact of the dopant.
Making the Right Choice for Your Experiment
To ensure your H2-TPR results are valid and reproducible, align your equipment choice with your specific analytical goals:
- If your primary focus is quantifying active sites: Rely on the inertness of quartz to ensure 100% of hydrogen consumption is attributed to CuO and Cu2(OH)3NO3 reduction.
- If your primary focus is studying promoter effects: Use the U-shaped design to ensure the high sensitivity required to detect the specific shifts caused by cerium doping.
The correct reactor vessel is the invisible baseline that transforms raw data into a reliable chemical mechanism.
Summary Table:
| Feature | Advantage for H2-TPR | Benefit to Data Quality |
|---|---|---|
| Quartz Material | Chemically inert up to 500°C+ | Eliminates "ghost" hydrogen consumption peaks |
| Thermal Stability | Resists warping under heat | Ensures consistent volume and pressure during sweeps |
| U-Shape Design | Promotes uniform gas flow | Guarantees total exposure of the adsorbent bed |
| High Sensitivity | Minimizes background noise | Resolves subtle shifts from Cerium-doping effects |
Maximize Your Analytical Precision with KINTEK
Don't let subpar reactor materials skew your critical H2-TPR data. KINTEK provides high-performance laboratory solutions backed by expert R&D and manufacturing. Our U-shaped quartz reactors and high-temperature systems are engineered for the total chemical inertness and thermal integrity required to resolve complex reduction mechanisms in catalysts like Cu–Ce/HZSM-5.
Whether you need Muffle, Tube, Rotary, Vacuum, or CVD systems, KINTEK offers fully customizable lab furnaces tailored to your unique research needs. Ensure your results are valid and reproducible with equipment built for excellence.
Ready to upgrade your characterization capabilities?
Contact KINTEK Today to Discuss Your Custom Solution
Visual Guide
References
- Zhiyuan Liu, Guoqiang Huang. Acid-modified Cu–Ce/HZSM-5 adsorbent removes trace phosphorus impurities from recycled hydrogen during polysilicon production. DOI: 10.1039/d5ra01322d
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
People Also Ask
- Why is an alumina crucible necessary when synthesizing U0.92Mn3Si2C inside a quartz tube? Ensure Vessel Integrity
- Why are high-purity alumina crucibles used for LLZO sintering? Master Lithium Volatility Control
- What is the technical role of a magnetic stirring hot plate in synthesis? Optimize Cobalt Oxide Nanoparticle Quality
- Why is the use of high-vacuum pump groups critical for photothermal catalytic chamber pre-treatment?
- How does the SOM method enhance titanium alloy purity? The Power of Solid Electrolyte Tubes
- What role does a precision drying oven play in the pre-treatment of Bi-Fe oxide powders? Safeguard Your Nano-Morphology
- Why is zirconia grinding media preferred for NN-10ST ceramic powders? Ensure Purity & Dielectric Performance
- What are the different grades of Alumina ceramic and how do they differ? Choose the Right Grade for Your Needs



















