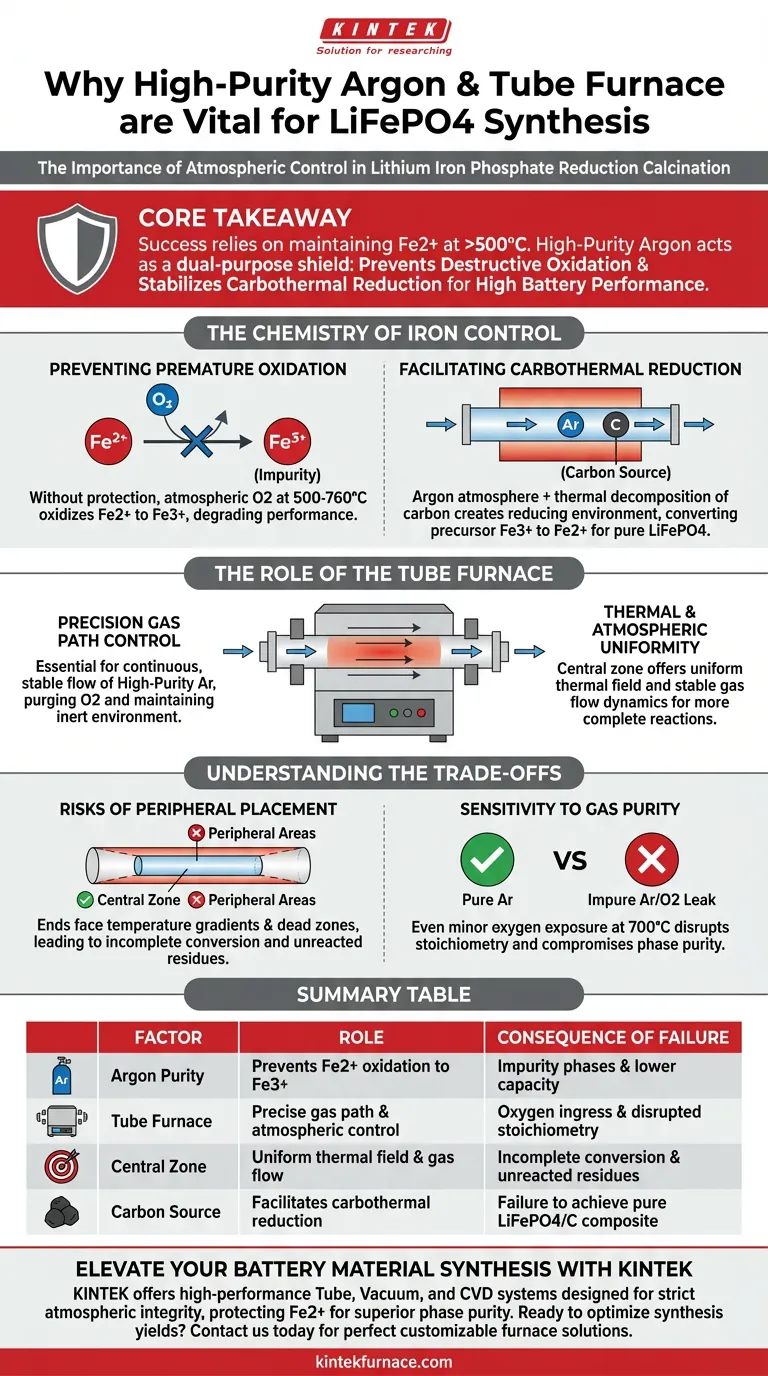
High-purity argon protection is strictly necessary during the reduction calcination of lithium iron phosphate (LiFePO4) to strictly control the oxidation state of iron. It creates a barrier against atmospheric oxygen while simultaneously supporting the chemical reduction required to synthesize highly active electrochemical materials.
Core Takeaway Success in synthesizing LiFePO4 relies on maintaining the iron in a divalent (Fe2+) state at temperatures exceeding 500°C. High-purity argon acts as a dual-purpose shield: it prevents the destructive oxidation of iron into impurity phases and stabilizes the carbothermal reduction process essential for high battery performance.

The Chemistry of Iron Control
Preventing Premature Oxidation
The fundamental challenge in synthesizing lithium iron phosphate is the sensitivity of iron at high temperatures (500-760°C).
Without protection, atmospheric oxygen reacts with the material. This causes the desired divalent iron (Fe2+) to oxidize into trivalent iron (Fe3+).
The presence of Fe3+ results in impurity phases. These impurities significantly degrade the electrochemical activity and capacity of the final battery material.
Facilitating Carbothermal Reduction
The argon atmosphere does more than just exclude oxygen; it enables necessary chemical changes.
During calcination, added carbon sources (such as glucose) undergo thermal decomposition.
This decomposition creates a reducing atmosphere within the tube furnace. This environment ensures that any precursor Fe3+ is successfully reduced to the correct Fe2+ state.
This process enables the synthesis of a pure LiFePO4/carbon composite with superior phase purity.
The Role of the Tube Furnace
Precision Gas Path Control
A standard oven cannot maintain the strict atmospheric conditions required for this reaction.
A tube furnace is essential because it is equipped with a high-precision gas path control system.
This system ensures a continuous, stable flow of high-purity argon. This constantly purges oxygen and maintains the inert environment throughout the entire calcination cycle.
Thermal and Atmospheric Uniformity
The physical location of the sample within the furnace is critical for reaction consistency.
The central zone of the tube furnace offers the most uniform thermal field. It also provides the most stable gas flow dynamics.
Precursors placed in this center zone undergo more complete reactions. This leads to higher yields and minimal impurities.
Understanding the Trade-offs
The Risks of Peripheral Placement
While the tube furnace enables control, it is not uniform across its entire length.
Materials placed in the peripheral areas (near the tube ends) face significant risks.
These areas suffer from temperature gradients and "dead zones" in the gas flow.
This results in incomplete precursor conversion. You will likely find unreacted residues or impurity phases in materials processed outside the central zone.
Sensitivity to Gas Purity
The process is intolerant of low-quality gas inputs.
Using argon with trace impurities or failing to seal the system allows oxygen ingress.
Even minor oxygen exposure at 700°C disrupts the stoichiometry. This irreversibly compromises the phase purity of the lithium iron phosphate.
Making the Right Choice for Your Goal
To ensure the successful synthesis of LiFePO4, you must prioritize equipment precision and protocol.
- If your primary focus is Phase Purity: Ensure your tube furnace utilizes a high-precision gas control system to maintain a strictly inert argon atmosphere, preventing Fe2+ oxidation.
- If your primary focus is Yield Consistency: Confine your sample placement to the central zone of the furnace to avoid thermal gradients and gas flow dead zones.
Ultimately, the electrochemical power of your final material is dictated by the integrity of the inert atmosphere during the reduction phase.
Summary Table:
| Factor | Role in LiFePO4 Synthesis | Consequence of Failure |
|---|---|---|
| Argon Purity | Prevents Fe2+ oxidation to Fe3+ | Impurity phases & lower capacity |
| Tube Furnace | Precise gas path & atmospheric control | Oxygen ingress & disrupted stoichiometry |
| Central Zone | Uniform thermal field & gas flow | Incomplete conversion & unreacted residues |
| Carbon Source | Facilitates carbothermal reduction | Failure to achieve pure LiFePO4/C composite |
Elevate Your Battery Material Synthesis with KINTEK
Precision is paramount in the reduction calcination of lithium iron phosphate. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Vacuum, and CVD systems designed to maintain the strict atmospheric integrity your research demands. Our customizable lab high-temp furnaces ensure optimal gas path control and thermal uniformity, protecting your Fe2+ state for superior phase purity.
Ready to optimize your synthesis yields? Contact us today to find the perfect customizable furnace solution for your unique laboratory needs.
Visual Guide
References
- Guangqiang Ma, Dongying Ju. Effect of impurities in FePO4 raw materials on the performance of LiFePO4 cathode materials. DOI: 10.1038/s41598-025-99729-8
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- Why is a high-precision gas flow control system required for vermiculite heat treatment? Ensure Perfect Atmosphere
- What role does a quartz tube furnace play in the carbonization of nitrogen-doped carbon? Optimize Your Material Synthesis
- What core functions does an argon atmosphere tube furnace perform? Optimize Al-PTFE FGM Sintering
- What recent improvements have been made to lab tube furnaces? Unlock Precision, Automation & Safety
- How does a high-temperature tube furnace facilitate HfOC/SiOC pyrolysis? Mastering Polymer-to-Ceramic Transition
- What materials are used for a tube furnace heating chamber? Optimize for temperature, purity, and durability.
- How does a split tube furnace compare to non-split tube furnaces? Choose the Right Furnace for Your Lab
- What role does a vacuum tube furnace play in AlCrSiWN coating annealing? Enhance Stability and Hardness



















