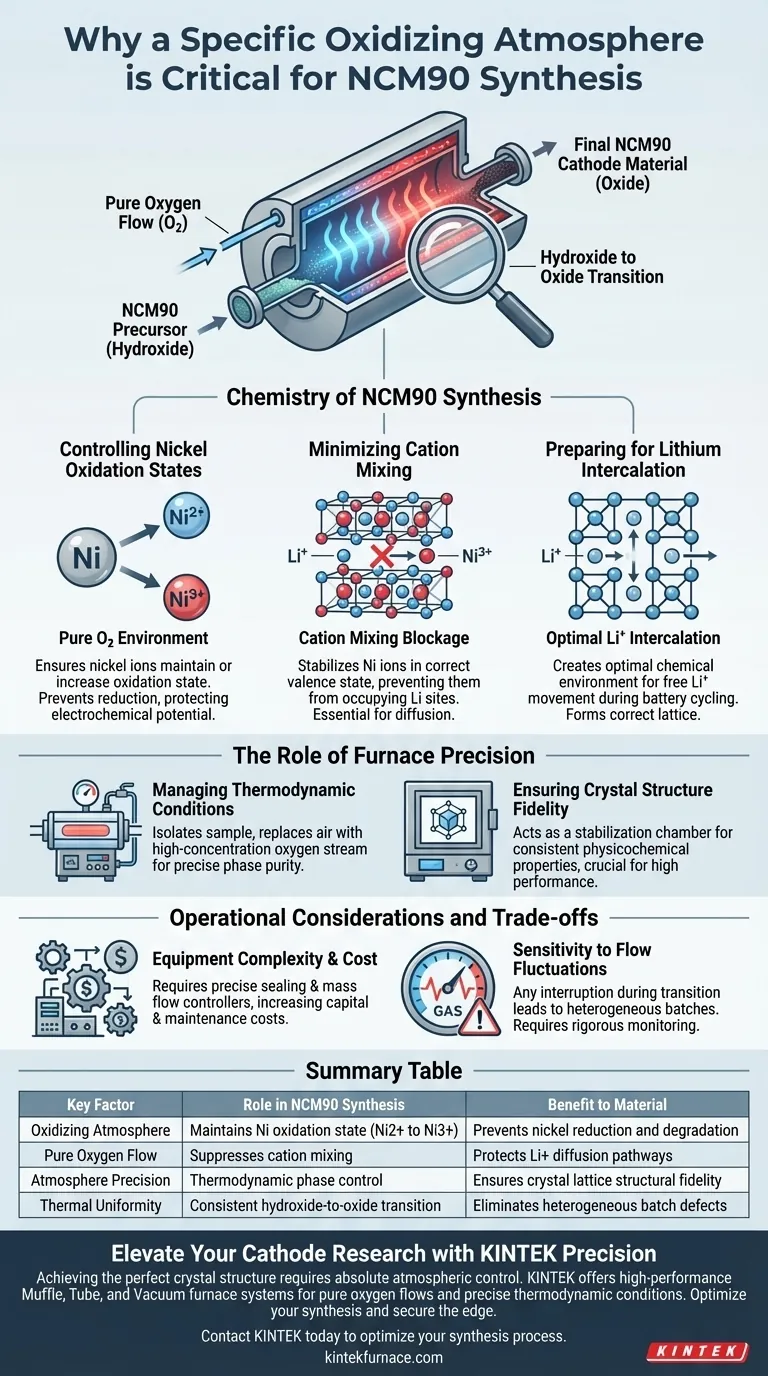
A specific oxidizing atmosphere is critical during the initial stages of NCM90 synthesis to strictly control the oxidation state of nickel ions. As the precursor transitions from a hydroxide to an oxide at lower temperatures, a pure oxygen flow prevents the reduction of nickel, ensuring the structural integrity required for high-performance battery materials.
By maintaining a pure oxygen environment, high-temperature furnaces facilitate the proper oxidation of nickel while suppressing cation mixing. This precise atmospheric control is the prerequisite for establishing the correct crystal lattice structure needed for efficient lithium intercalation.

The Chemistry of NCM90 Synthesis
Controlling Nickel Oxidation States
During the initial heating phase, the precursor material undergoes a chemical transformation from a hydroxide to an oxide. This is a vulnerable stage where the chemical stability of the material is in flux.
Pure oxygen flow is mandated to ensure that nickel ions maintain or increase their oxidation state. Without this oxygen-rich environment, nickel is prone to reduction, which degrades the electrochemical potential of the final cathode material.
Minimizing Cation Mixing
A major challenge in high-nickel cathodes like NCM90 is cation mixing, where nickel ions incorrectly occupy sites meant for lithium ions. This disorder blocks the diffusion pathways required for battery operation.
The primary reference indicates that precise control of the oxidizing atmosphere significantly reduces this phenomenon. By stabilizing the nickel ions in their correct valence state, the furnace environment preserves the layered structure necessary for performance.
Preparing for Lithium Intercalation
The ultimate goal of this atmospheric control is to create an optimal chemical environment for the intercalation of lithium ions.
If the oxidation environment is insufficient during the hydroxide-to-oxide transition, the resulting crystal lattice will be defective. A pure oxygen atmosphere ensures the lattice forms correctly, allowing lithium ions to move freely in and out of the structure during battery cycling.
The Role of Furnace Precision
Managing Thermodynamic Conditions
Laboratory tube and muffle furnaces are essential because they allow reactions to occur under specific thermodynamic conditions.
Standard heating without atmosphere control cannot guarantee the phase purity required for NCM90. These furnaces enable the isolation of the sample from ambient air, replacing it with the necessary high-concentration oxygen stream.
Ensuring Crystal Structure Fidelity
As noted in the supplementary references, precise thermal and atmosphere management is critical for obtaining materials with specific crystal structures.
For NCM90, the difference between a high-performance cathode and a failed batch often lies in the consistency of the atmosphere. The furnace acts as a stabilization chamber, ensuring the physicochemical properties are uniform throughout the sample.
Operational Considerations and Trade-offs
Equipment Complexity and Cost
While a pure oxygen atmosphere is chemically necessary, it introduces significant equipment complexity.
Using tube or muffle furnaces with gas flow capabilities requires precise sealing mechanisms and mass flow controllers. This increases the capital cost and maintenance requirements compared to standard air-calcination processes.
Sensitivity to Flow Fluctuations
The process is highly sensitive to the consistency of the oxygen flow.
Any interruption or fluctuation in the atmosphere supply during the critical hydroxide-to-oxide transition can lead to heterogeneous batches. This requires rigorous monitoring systems to ensure the atmosphere remains constant throughout the multi-hour synthesis process.
Optimizing Your Synthesis Strategy
To achieve the best results with NCM90 synthesis, align your equipment choice with your specific quality metrics:
- If your primary focus is maximizing discharge capacity: Prioritize a furnace with high-precision gas flow controllers to minimize cation mixing, as this directly correlates to available lithium pathways.
- If your primary focus is crystal structural stability: Ensure your furnace provides exceptional thermal uniformity alongside oxygen flow to prevent localized reduction of nickel ions during the phase transition.
Mastering the oxidizing atmosphere is not merely a procedural step; it is the fundamental control lever for engineering high-performance NCM90 cathodes.
Summary Table:
| Key Factor | Role in NCM90 Synthesis | Benefit to Material |
|---|---|---|
| Oxidizing Atmosphere | Maintains Ni oxidation state (Ni2+ to Ni3+) | Prevents nickel reduction and degradation |
| Pure Oxygen Flow | Suppresses cation mixing | Protects Li+ diffusion pathways |
| Atmosphere Precision | Thermodynamic phase control | Ensures crystal lattice structural fidelity |
| Thermal Uniformity | Consistent hydroxide-to-oxide transition | Eliminates heterogeneous batch defects |
Elevate Your Cathode Research with KINTEK Precision
Achieving the perfect crystal structure for NCM90 requires more than just heat—it requires absolute atmospheric control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, and Vacuum furnace systems specifically designed to handle pure oxygen flows and precise thermodynamic conditions.
Whether you are scaling up solid-state synthesis or refining lab-scale prototypes, our customizable high-temp solutions ensure the structural fidelity your high-nickel materials demand. Contact KINTEK today to optimize your synthesis process and secure the competitive edge in battery material innovation.
Visual Guide
References
- Yucheng Wu, Jin Xie. Enabling uniform lithiation in solid-state synthesis by preventing pre-matured surface grain coarsening through grain boundary engineering. DOI: 10.1039/d5sc00271k
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the primary functions of industrial-grade constant temperature furnaces for NiCoCrAlY oxidation kinetics?
- What kind of atmosphere is typically used in low vacuum atmosphere furnaces? Optimize Your Heat Treatment with Inert Gases
- What are the primary industrial applications of atmosphere furnaces? Essential for High-Temp Material Processing
- Why is a high-precision furnace essential for CZTSSe thin films? Prevent Phase Decomposition and Amorphization
- How does an atmosphere furnace contribute to research and development? Unlock Advanced Material Innovation
- Why use multi-stage temperature control for REBCO pyrolysis? Prevent 75% Shrinkage Cracks and Ensure Film Density
- What is an atmosphere box furnace and its primary applications? Essential for High-Temperature Controlled Environments
- What functions does a box resistance furnace perform for ultra-high-strength spring steel? Expert Heat Treatment Guide



















