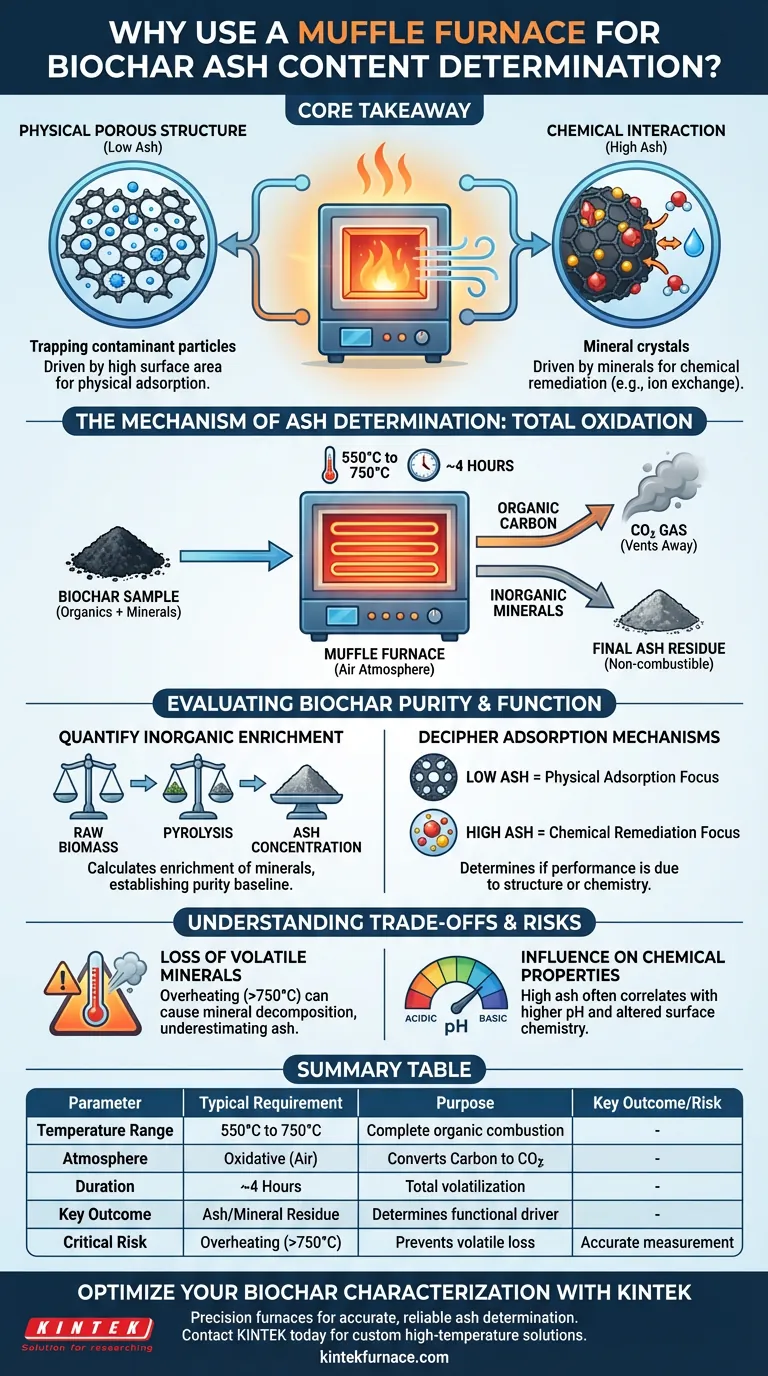
A muffle furnace is the primary instrument for determining ash content because it creates the controlled, high-temperature oxidative environment necessary to completely remove all organic material from the biochar. By heating samples to temperatures typically between 550°C and 750°C in an air atmosphere for several hours, the furnace ensures that the carbon structure is fully combusted and volatilized, leaving behind only the inorganic mineral residue for accurate measurement.
Core Takeaway Determining ash content is a critical diagnostic step that reveals whether a biochar’s effectiveness is driven by its physical porous structure or by chemical interactions with its mineral components. The muffle furnace isolates these inorganic elements by systematically eliminating the organic carbon matrix.

The Mechanism of Ash Determination
Complete Combustion of Organics
The primary function of the muffle furnace in this context is total oxidation. Biochar consists of a carbon-rich skeleton and inorganic minerals.
To measure the minerals (ash), the carbon skeleton must be destroyed. The furnace provides a consistent oxygen-rich environment at high heat, converting the organic carbon into gases (like CO2) that vent away, isolating the non-combustible material.
Precise Temperature Regulation
Ash determination requires specific thermal parameters to ensure accuracy. The muffle furnace maintains steady temperatures, generally around 550°C to 750°C, for extended periods (often 4 hours).
This sustained heat ensures that the combustion is complete throughout the entire sample mass, preventing "unburned" carbon from skewing the weight of the final ash residue.
Evaluating Biochar Purity and Function
Quantifying Inorganic Enrichment
Raw biomass, particularly waste fiber, contains varying levels of inorganic additives or natural minerals. During the initial pyrolysis (making the biochar), these minerals become concentrated as the biomass loses mass.
Using a muffle furnace allows you to calculate the enrichment of these inorganic substances. This creates a baseline for purity, confirming how much of the final product is active carbon versus mineral filler.
Deciphering Adsorption Mechanisms
This is the most critical technical insight provided by ash analysis. Understanding the ratio of ash to carbon helps engineers determine how the biochar actually works.
If ash content is low, the biochar's performance (such as pollutant removal) is likely driven by its physical porous structure. If ash content is high, performance may be driven by chemical interactions with the mineral components.
Understanding the Trade-offs
Loss of Volatile Minerals
While the muffle furnace is the standard, high temperatures can cause the volatilization of certain unstable inorganic compounds.
If the furnace temperature is set too high (e.g., exceeding 750°C for certain biomass types), you risk underestimating the ash content because some minerals may decompose and escape as gas.
Influence on Chemical Properties
The ash content derived from this process is not inert; it serves as a proxy for pH and surface chemistry.
A high ash content often correlates with higher pH levels and altered electrostatic interactions with water. It is vital to interpret muffle furnace data in the context of these chemical shifts, rather than just as a weight percentage.
Making the Right Choice for Your Goal
To utilize muffle furnace data effectively, align your analysis with your project's end goal:
- If your primary focus is Physical Adsorption (Porous Structure): Look for low ash content results, as this indicates a high surface area of pure carbon available for trapping contaminants.
- If your primary focus is Chemical Remediation (Mineral Interaction): Look for higher ash content, which suggests the presence of inorganic minerals that can facilitate ion exchange or precipitation reactions.
The muffle furnace does not merely measure waste; it reveals the fundamental balance between the organic framework and the inorganic engine of your biochar.
Summary Table:
| Parameter | Typical Requirement | Purpose in Biochar Analysis |
|---|---|---|
| Temperature Range | 550°C to 750°C | Ensures complete combustion of organic carbon skeleton |
| Atmosphere | Oxidative (Air) | Facilitates conversion of carbon to CO2 gas |
| Duration | ~4 Hours | Guarantees total volatilization throughout sample mass |
| Key Outcome | Ash/Mineral Residue | Determines if biochar works via physical or chemical means |
| Critical Risk | Overheating (>750°C) | Prevents loss of volatile minerals for accurate measurement |
Optimize Your Biochar Characterization with KINTEK
Precision in ash determination starts with uniform heat and reliable atmosphere control. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your laboratory's unique high-temperature needs. Whether you are quantifying inorganic enrichment or optimizing porous structures, our furnaces provide the stability your research demands.
Ready to elevate your material analysis? Contact KINTEK today for a custom solution!
Visual Guide
References
- Robert Wolski, Robert Pietrzak. Methylene Blue and Rhodamine B Dyes’ Efficient Removal Using Biocarbons Developed from Waste. DOI: 10.3390/molecules29174022
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How should temperature be managed when using a muffle furnace? Master Precise Control for Safety and Accuracy
- What optional features are available for box furnaces? Customize for Your Lab's Unique Needs
- How does an industrial-grade box-type high-temperature furnace contribute to the formation of the NaY(WO4)2 crystal phase?
- Why is a box muffle furnace utilized for the pre-sintering of bauxite residue green pellets at 1150°C?
- What materials are used in muffle furnace construction? Key Materials for High-Temp Performance
- What key components are used in vacuum muffle furnaces to ensure precise gas dispersion? Discover the MFC and BPR System
- Why is a muffle furnace with multi-stage programmable heating control necessary for sol-gel magnesium oxide synthesis?
- Why is a high-temperature box resistance furnace essential for failure analysis? Master TGO Growth and Isothermal Testing



















