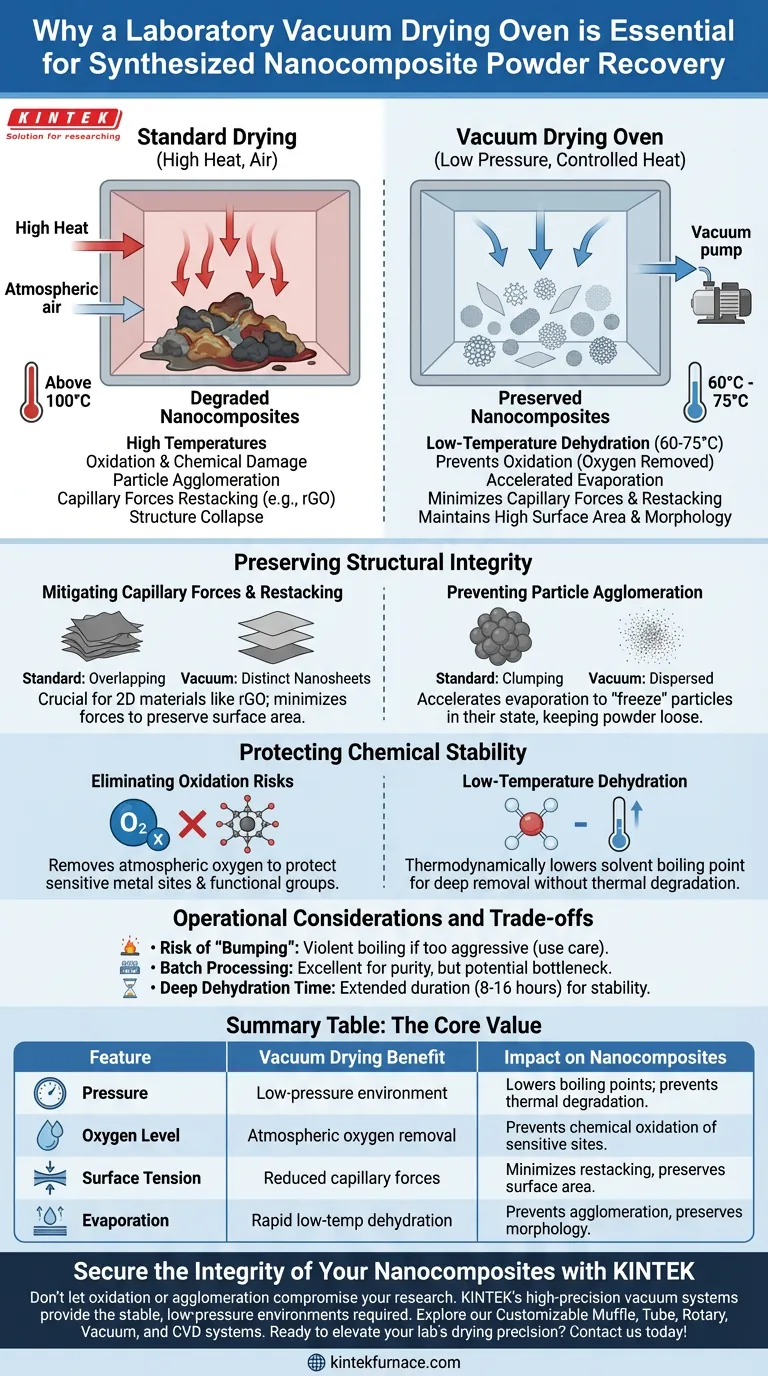
A laboratory vacuum drying oven is essential for nanocomposite recovery because it creates a low-pressure environment that forces moisture and residual solvents to evaporate rapidly at significantly reduced temperatures. This process is critical for preventing the physical degradation and chemical oxidation that frequently destroy delicate nanomaterials during standard thermal drying.
The Core Value of Vacuum Drying Nanocomposites are highly sensitive to heat and surface tension. A vacuum drying oven circumvents these dangers by lowering the solvent boiling point, allowing for deep dehydration without the high temperatures that cause oxidation, particle agglomeration, or the collapse of nanostructures.
Preserving Structural Integrity
Mitigating Capillary Forces and Restacking
For two-dimensional materials, such as reduced graphene oxide (rGO), the drying phase is perilous. In standard drying, the evaporation of liquid creates strong capillary forces.
These forces pull nanosheets together, leading to severe overlapping and restacking. A vacuum environment minimizes these capillary forces, ensuring the nanosheets remain distinct and preserving the material's high surface area.
Preventing Particle Agglomeration
When drying synthesized powders, such as silver nanoparticles or MnMgPO4 composites, protecting the specific morphology is vital.
High temperatures and slow evaporation rates often cause these fine particles to clump together (agglomerate). Vacuum drying accelerates evaporation at low temperatures, "freezing" the particles in their dispersed state and ensuring the final powder remains loose and easy to grind.
Protecting Chemical Stability
Eliminating Oxidation Risks
Many nanocomposites contain active metal sites or functional groups that are sensitive to oxygen. Standard ovens circulate hot air, which accelerates oxidation and can ruin the chemical purity of materials like tin (Sn) anodes or fluoride-based cathodes.
By operating under a vacuum, the oven removes atmospheric oxygen. This protects sensitive components, such as active nitro functional groups in catalysts, preventing premature decomposition that would otherwise occur in a heated, oxygen-rich environment.
Low-Temperature Dehydration
The fundamental advantage of this equipment is thermodynamic: lowering pressure lowers the boiling point of water and solvents.
This allows for the complete removal of stubborn solvents (like anhydrous ethanol) and moisture from deep within catalyst pores at temperatures as low as 60°C to 75°C. This avoids the phase changes or thermal degradation that often plague materials dried at higher temperatures (e.g., above 100°C).
Operational Considerations and Trade-offs
While vacuum drying is superior for preservation, it requires careful operational control compared to standard blast ovens.
- Risk of "Bumping": If the vacuum is applied too aggressively to a wet slurry, the solvent may boil violently (bump). This can splatter the sample inside the chamber, leading to material loss.
- Batch Processing: Unlike some continuous drying methods, vacuum ovens are typically batch-process tools. This is excellent for purity but may introduce bottlenecks if high-throughput processing is required.
- Deep Dehydration Time: While the boiling point is lower, removing the final traces of capillary water from deep pores still requires extended duration (often 8–16 hours) to ensure structural stability during subsequent sintering stages.
Making the Right Choice for Your Goal
To maximize the quality of your nanocomposite recovery, tailor your drying strategy to your specific material constraints.
- If your primary focus is preserving morphology (e.g., Nanosheets/rGO): Prioritize vacuum drying to eliminate the capillary forces that cause restacking and surface area loss.
- If your primary focus is chemical purity (e.g., Oxygen-sensitive metals): Use the vacuum feature to exclude oxygen, preventing the hydrolysis or oxidation of active sites.
- If your primary focus is removing deep-pore solvents: Utilize the lowered boiling point to drive out trapped ethanol or water without thermally shocking the material structure.
Vacuum drying is not just a method of moisture removal; it is a preservation technique that secures the structural and chemical identity of your synthesized materials.
Summary Table:
| Feature | Vacuum Drying Benefit | Impact on Nanocomposites |
|---|---|---|
| Pressure | Low-pressure environment | Lowers boiling points; prevents thermal degradation. |
| Oxygen Level | Atmospheric oxygen removal | Prevents chemical oxidation of sensitive metal sites. |
| Surface Tension | Reduced capillary forces | Minimizes nanosheet restacking and preserves surface area. |
| Evaporation | Rapid low-temp dehydration | Prevents particle agglomeration and preserves morphology. |
Secure the Integrity of Your Nanocomposites with KINTEK
Don't let oxidation or agglomeration compromise your research. KINTEK’s high-precision vacuum systems are engineered to provide the stable, low-pressure environments required for delicate material recovery.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, CVD systems, and other lab high-temp furnaces, all customizable for your unique material needs. Whether you are working with graphene oxide, silver nanoparticles, or sensitive catalysts, our equipment ensures deep dehydration without structural collapse.
Ready to elevate your lab's drying precision? Contact us today to find your custom solution!
Visual Guide
References
- Aliaa Abdelfattah, Ahmed M. Selim. Mechanochemical enhancement in electrode materials via silver-embedded reduced graphene oxide and cobalt oxide nanostructure for supercapacitor applications. DOI: 10.1007/s11581-024-05385-9
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What are the maintenance requirements for a vacuum furnace when not in use? Protect Your Investment with Proper Storage
- What is the advantage of computer-controlled processes in vacuum furnaces? Achieve Perfect Repeatability for High-Performance Applications
- How does vacuum sintering compare to traditional smelting methods? Discover Key Differences for Your Manufacturing Needs
- How does a vacuum drying oven provide superior results for Cs3Cu2I5:Tb precipitates? Preserve Your Scintillation Quality
- What are the functions of a heat shield in a vacuum sintering furnace? Essential for Thermal Control and Efficiency
- What are the advantages of vacuum heat treatment? Achieve Superior Cleanliness and Performance for Critical Parts
- What factors should be considered when choosing a vacuum furnace model? Key Insights for Optimal Performance
- How does vacuum carburizing improve surface quality? Achieve Clean, High-Strength Parts



















