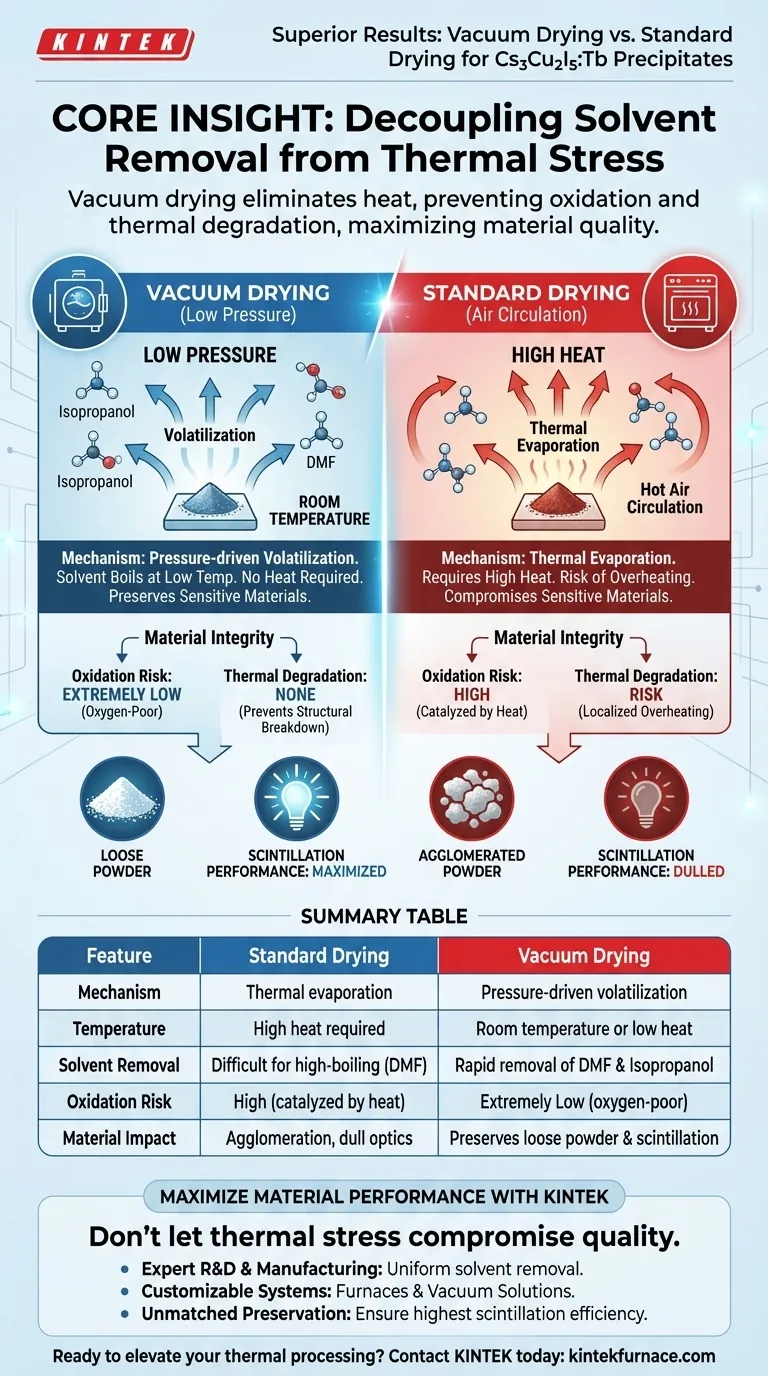
Vacuum drying provides superior results by decoupling solvent removal from thermal stress, allowing for the preservation of delicate material properties that standard heating destroys.
For Cs3Cu2I5:Tb precipitates, a vacuum drying oven works by drastically reducing environmental pressure. This lowers the boiling point of residual solvents like isopropanol and dimethylformamide (DMF), causing them to volatilize rapidly at room temperature. By eliminating the need for high heat, this method prevents the oxidation and thermal degradation inherent in standard drying, directly preserving the material's critical scintillation performance.
Core Insight: The superiority of vacuum drying lies in its ability to remove stubborn solvents without thermal energy. By substituting heat with low pressure, you eliminate the primary cause of material degradation—oxidation and thermal shock—thereby maximizing the optical and physical quality of the final product.

The Mechanism of Low-Pressure Drying
Volatilization Without Heat
Standard drying relies on heat to evaporate moisture. Vacuum drying alters the physics of the environment.
By creating a negative pressure environment, the vacuum oven significantly lowers the boiling point of liquids. This allows solvents to turn into gas and evaporate efficiently, even while the material remains at room temperature.
Targeting Stubborn Solvents
Processing Cs3Cu2I5:Tb involves solvents such as isopropanol and dimethylformamide (DMF).
DMF, in particular, can be difficult to remove under standard atmospheric conditions without raising temperatures significantly. Vacuum drying facilitates the rapid removal of these heavy solvents without requiring the temperature spikes that damage the precipitate.
Preserving Material Integrity
Preventing Thermal Degradation
Metal halide materials are often thermally sensitive.
Standard ovens force you to choose between incomplete drying and thermal damage. Vacuum drying removes this trade-off. By drying at room temperature or very low temperatures, you avoid the structural breakdown of the metal halide lattice that occurs during heated drying.
Eliminating Oxidation Risks
Heat acts as a catalyst for oxidation, which ruins the purity of the material.
A vacuum environment is naturally oxygen-poor. This prevents the chemical reaction between the material and atmospheric oxygen. This is critical for maintaining the high electrical conductivity (in applicable contexts) and structural integrity of the precipitates.
Protecting Scintillation Performance
The ultimate goal of processing Cs3Cu2I5:Tb is usually its application in scintillation (emitting light when struck by radiation).
Thermal degradation and oxidation dull these optical properties. By utilizing vacuum drying to maintain a pristine, unoxidized structure, you ensure the final material retains maximum scintillation efficiency.
Understanding the Trade-offs: Vacuum vs. Standard Heating
The Limits of Standard Air Circulation
Constant temperature ovens (standard heating) utilize internal air circulation.
While this promotes thermal uniformity and is excellent for removing pinholes in film layers, it relies on heating the air. For sensitive precipitates, this heat promotes localized overheating and surface degradation that vacuum drying avoids.
Structural Considerations
Standard heating can sometimes cause powders to cake or agglomerate due to uneven evaporation rates.
Vacuum drying, by contrast, tends to maintain a loose powder structure. Because the solvent is pulled out rapidly and uniformly via pressure rather than slowly via heat transfer, the resulting powder is often easier to load and process in subsequent pyrolysis or synthesis steps.
Making the Right Choice for Your Goal
To ensure optimal processing of your Cs3Cu2I5:Tb precipitates, align your drying method with your specific quality metrics.
- If your primary focus is Scintillation Efficiency: Prioritize vacuum drying at room temperature to strictly avoid thermal degradation and preserve optical properties.
- If your primary focus is Solvent Purity: Use vacuum drying to ensure the complete volatilization of DMF and isopropanol without triggering oxidation.
- If your primary focus is Powder Handling: Rely on vacuum drying to produce a loose, non-agglomerated powder structure that is easy to utilize in subsequent manufacturing steps.
Summary: Vacuum drying is not just a faster drying method; it is a preservation technique that ensures your chemical precipitates retain the high-performance characteristics usually lost to thermal processing.
Summary Table:
| Feature | Standard Drying (Air Circulation) | Vacuum Drying (Low Pressure) |
|---|---|---|
| Mechanism | Thermal evaporation via heat | Pressure-driven volatilization |
| Temperature | High heat required | Room temperature or low heat |
| Solvent Removal | Difficult for high-boiling solvents (DMF) | Rapid removal of DMF & Isopropanol |
| Oxidation Risk | High (catalyzed by heat) | Extremely Low (oxygen-poor environment) |
| Material Impact | Risk of agglomeration & dull optics | Preserves loose powder & scintillation |
| Best For | General moisture removal | Sensitive chemical precipitates |
Maximize Your Material Performance with KINTEK
Don't let thermal stress compromise your research or production quality. KINTEK provides industry-leading high-temperature and vacuum solutions tailored for sensitive materials like metal halide precipitates.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our specialized vacuum ovens ensure uniform solvent removal without oxidation.
- Customizable Systems: From Muffle and Tube furnaces to Rotary, Vacuum, and CVD systems, we build to your unique laboratory needs.
- Unmatched Preservation: Ensure the highest scintillation efficiency and structural integrity for your precipitates.
Ready to elevate your thermal processing? Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Haifeng Chen. Study on rare-earth element-doped copper halides. DOI: 10.54254/2977-3903/2025.23781
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How does a high-temperature vacuum furnace convert PDA into nitrogen-doped carbon shells? Master Carbonization Control
- Why is a vacuum drying oven set to 70 °C for g-C3N4/Bi2WO6? Optimize Your Photocatalyst Post-Processing
- How does heat transfer occur in a high-temperature vacuum furnace, and what factors influence its efficiency? Master Radiant Heat Control
- How do multiple-chamber vacuum furnaces improve energy efficiency? Cut Costs with Continuous Heating
- How does a modern pressure sintering furnace operate? Unlock High-Density Materials with Precision
- What is the function of a vacuum furnace in phosphor synthesis? Achieve Pure Tb3+/Ce3+ Doped Wollastonite Materials
- What are the advantages of using a vacuum dryer for cerium oxide nanoparticles? Preserve Integrity & Prevent Oxidation
- Why must the diffusion bonding of ODS steel and nickel alloys be done in a vacuum furnace? Achieve Pore-Free Integrity



















