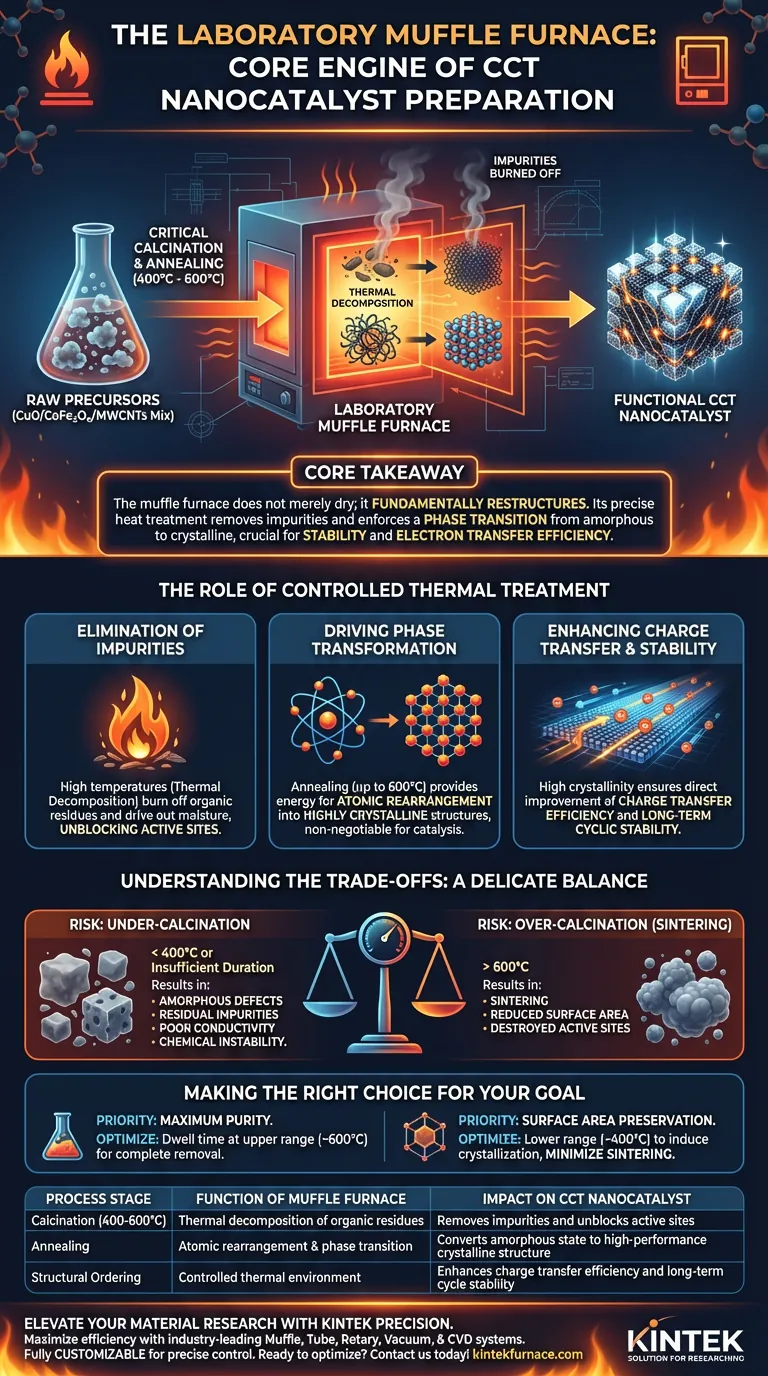
The laboratory muffle furnace is the primary engine for the critical calcination and annealing stages in the synthesis of CuO/CoFe2O4/MWCNTs (CCT) nanocatalysts. By maintaining a strictly controlled thermal environment between 400°C and 600°C, the furnace drives the essential chemical and physical transformations required to convert raw precursors into a functional, high-performance catalytic material.
Core Takeaway The muffle furnace does not merely dry the material; it fundamentally restructures it. Its precise heat treatment removes impurities and enforces a phase transition from amorphous to crystalline, which is the deciding factor in the catalyst's stability and electron transfer efficiency.
The Role of Controlled Thermal Treatment
The preparation of CCT nanocatalysts is not simply about mixing ingredients; it is about engineering a specific crystal structure. The muffle furnace acts as the environment where this structural engineering takes place.
Elimination of Impurities
Raw precursor materials often contain residual water molecules and organic impurities that can inhibit catalytic activity.
The muffle furnace utilizes high temperatures to trigger thermal decomposition. This effectively burns off organic residues and drives out moisture that would otherwise block active sites on the catalyst's surface.
Driving Phase Transformation
The initial components of the catalyst mixture often exist in an amorphous (disordered) state.
Through annealing at temperatures up to 600°C, the furnace provides the energy needed to rearrange atoms into a highly crystalline phase structure. This transition is non-negotiable for achieving the material properties required for advanced catalysis.
Enhancing Charge Transfer and Stability
A catalyst's efficiency depends heavily on how well it facilitates electron movement.
By ensuring high crystallinity, the muffle furnace directly improves the charge transfer efficiency of the final CCT composite. Furthermore, this structural ordering solidifies the material, granting it the long-term stability necessary to withstand repeated catalytic cycles without degrading.
Understanding the Trade-offs
While the muffle furnace is essential, the "more heat is better" approach is a common pitfall. The process requires a delicate balance.
The Risk of Over-Calcination
If the temperature exceeds the optimal range (above 600°C for this specific application), you risk sintering. This causes nanoparticles to clump together, drastically reducing the surface area and destroying the active sites you worked to create.
The Risk of Under-Calcination
Conversely, failing to reach the required temperature or duration results in incomplete phase transformation. This leaves the material with amorphous defects and residual impurities, leading to poor conductivity and chemically unstable catalysts.
Making the Right Choice for Your Goal
To maximize the performance of your CCT nanocatalysts, you must tailor the furnace parameters to your specific objectives.
- If your primary focus is maximum purity: Prioritize a dwell time at the upper end of the temperature window (near 600°C) to ensure the complete removal of stubborn organic impurities and water.
- If your primary focus is surface area preservation: Utilize the lower end of the effective temperature range (closer to 400°C) to induce crystallization while minimizing the risk of particle sintering.
The muffle furnace is not just a heater; it is a precision instrument that dictates the ultimate efficiency and lifespan of your nanocatalyst.
Summary Table:
| Process Stage | Function of Muffle Furnace | Impact on CCT Nanocatalyst |
|---|---|---|
| Calcination (400-600°C) | Thermal decomposition of organic residues | Removes impurities and unblocks active sites |
| Annealing | Atomic rearrangement & phase transition | Converts amorphous state to high-performance crystalline structure |
| Structural Ordering | Controlled thermal environment | Enhances charge transfer efficiency and long-term cycle stability |
Elevate Your Material Research with KINTEK Precision
Maximize the efficiency of your CCT nanocatalyst synthesis with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and world-class manufacturing, we provide Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of laboratory and industrial high-temperature applications.
Whether you require precise temperature control to prevent sintering or specialized atmospheric conditions for unique material properties, our furnaces are fully customizable to meet your exact research needs.
Ready to optimize your catalytic performance? Contact us today to find your perfect furnace solution!
Visual Guide
References
- Davis Varghese, M. Victor Antony Raj. Synergistic design of CuO/CoFe₂O₄/MWCNTs ternary nanocomposite for enhanced photocatalytic degradation of tetracycline under visible light. DOI: 10.1038/s41598-024-82926-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What function do muffle furnaces play in sulfonation-induced crosslinking? Master PP Precursor Transformation
- What industries commonly use industrial muffle furnaces? Unlock Precision Heating for Diverse Sectors
- What is the function of a high-temperature muffle furnace in the determination of ash and crude fiber? Expert Analysis
- Why is the intended application important when selecting a muffle furnace? Ensure Precision and Efficiency for Your Lab
- What precautions should be taken for the first use or after long-term shutdown of a muffle furnace? Ensure Safe, Reliable Operation from Day One
- What are the safety procedures for loading and unloading samples in a muffle furnace? Ensure Operator and Equipment Safety
- What is a muffle furnace and what are its primary uses? Unlock Precise High-Temp Solutions
- What are the key design differences between muffle furnaces and drying ovens? Choose the Right Tool for Your Lab



















