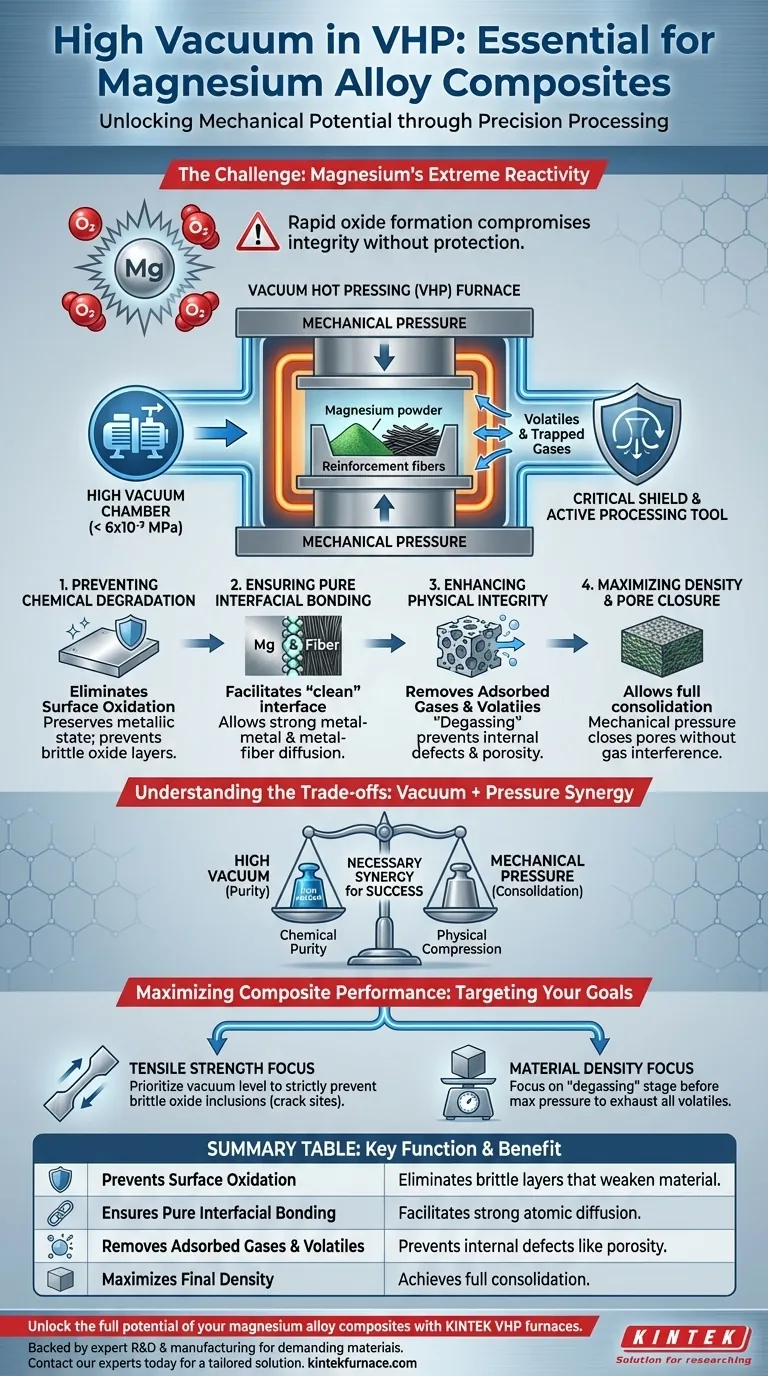
Magnesium’s extreme chemical reactivity makes a high vacuum environment non-negotiable during Vacuum Hot Pressing (VHP). The vacuum acts as a critical shield, reducing the oxygen content to negligible levels (e.g., 6 x 10^-3 MPa) to prevent the rapid formation of brittle oxides that would otherwise compromise the structural integrity of the composite.
The primary function of the high vacuum is to maintain surface purity and facilitate densification. By isolating the magnesium matrix from oxygen and actively extracting trapped gases, the vacuum ensures strong interfacial bonding between the metal and reinforcement phases, which is the determining factor for mechanical performance.

Preventing Chemical Degradation
Eliminating Surface Oxidation
Magnesium alloys, such as AZ31 and AZ91, are highly prone to oxidation, especially at the elevated temperatures required for sintering. Without a vacuum, oxygen reacts instantly with the magnesium surface.
This reaction forms oxide layers that are chemically stable but mechanically brittle. The high vacuum environment effectively isolates the material from oxygen, preserving the metallic state of the magnesium powder or plates throughout the heating process.
Ensuring Pure Interfacial Bonding
For a composite to possess high strength, the matrix (magnesium) must bond directly to the reinforcement (fibers or particles). Oxide layers act as a barrier to this bonding, preventing the necessary atomic diffusion between layers.
By preventing these impurity layers from forming, the vacuum facilitates a "clean" interface. This allows for the formation of pure metal-metal and metal-fiber connections, which are essential for transferring stress effectively within the composite.
Enhancing Physical Integrity
Removal of Adsorbed Gases and Volatiles
Raw materials, particularly powders, often carry adsorbed gases or volatile impurities on their surfaces. During the initial stages of sintering, these impurities are released.
The vacuum environment actively extracts these volatiles and exhausts residual gases trapped between stacked layers. If these gases were not removed, they would expand during heating, leading to internal defects.
Maximizing Density and Pore Closure
Achieving near-theoretical density is a primary goal of hot pressing. Residual gases trapped in microscopic gaps can prevent the material from compacting fully, leading to porosity.
The vacuum environment promotes "degassing," helping to expel air from these microscopic interface gaps. This allows the mechanical pressure of the VHP to effectively close pores, resulting in a dense, defect-free composite structure.
Understanding the Trade-offs
The Necessity of Mechanical Pressure
While a high vacuum is essential for chemical purity, it is rarely sufficient on its own to achieve full consolidation. Reference data suggests that vacuum must be combined with mechanical pressure to ensure success.
Mechanical pressure is required to forcibly disrupt any pre-existing oxide films that the vacuum cannot remove and to physically compress the material. Relying solely on vacuum without adequate pressure may result in poor atomic diffusion efficiency, even if the environment is oxygen-free.
Maximizing Composite Performance
To ensure you are utilizing the VHP process effectively for magnesium composites, consider your specific performance targets:
- If your primary focus is Tensile Strength: Prioritize the vacuum level to strictly prevent brittle oxide inclusions, which act as crack initiation sites.
- If your primary focus is Material Density: Focus on the "degassing" stage under vacuum before maximum pressure is applied to ensure all volatile impurities are exhausted from the powder mixture.
Ultimately, the high vacuum is not just a protective measure; it is an active processing tool that purifies interfaces to unlock the full mechanical potential of magnesium alloys.
Summary Table:
| Key Function of High Vacuum | Benefit for Magnesium Composite |
|---|---|
| Prevents Surface Oxidation | Eliminates brittle oxide layers that weaken the material. |
| Ensures Pure Interfacial Bonding | Facilitates strong atomic diffusion between matrix and reinforcement. |
| Removes Adsorbed Gases & Volatiles | Prevents internal defects like porosity by degassing the material. |
| Maximizes Final Density | Allows mechanical pressure to fully consolidate the material without gas interference. |
Unlock the full potential of your magnesium alloy composites with a precision VHP furnace from KINTEK.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, including customizable lab high-temperature furnaces designed for demanding materials like magnesium. Our solutions ensure the critical high-vacuum environment you need to prevent oxidation and achieve superior material density and strength.
Ready to enhance your research or production? Contact our experts today to discuss your specific application and receive a tailored solution.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- What are the key components of a vacuum hot press? Master Temperature, Pressure, and Atmosphere Control
- What are the advantages of a vacuum hot pressing sintering furnace for rare earth copper composites? Density & Purity
- What is a vacuum press used for? Achieve Flawless Bonding and Material Transformation
- What are the technical advantages of using Pulsed Current Sintering (PCS) for Ag2S1-xTex? Optimize Your Microstructure
- What are the processing advantages of SPS systems for LaFeO3 ceramics? Achieve High Density with Precision
- Why is vacuum press technology indispensable in modern metalworking? Unlock Precision and Quality in Metal Forming
- How does temperature control precision of a vacuum hot press affect SiC fiber/TB8 matrix? Optimize Interface Quality
- What is the core function of a flat tablet press in CSP? Achieve High-Pressure Densification for CaF2 Ceramics



















