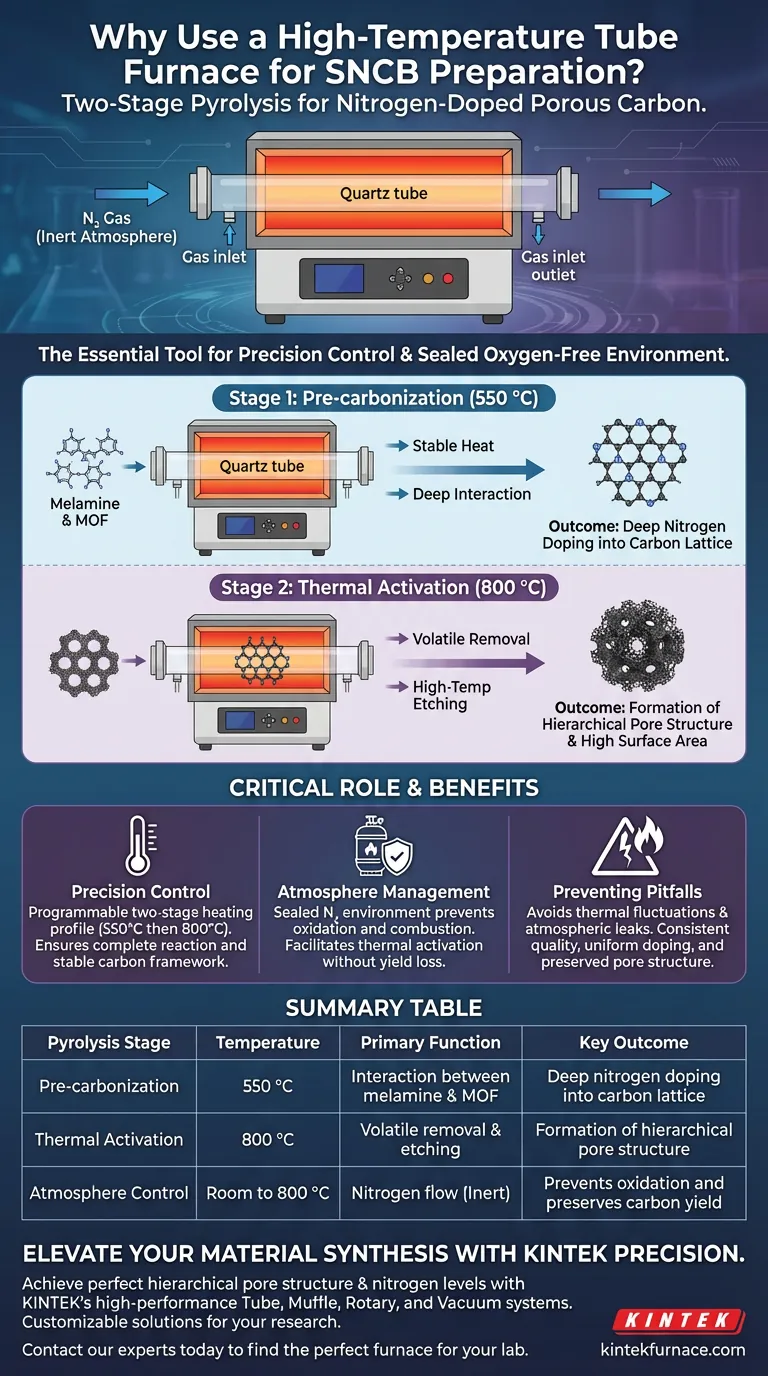
A high-temperature tube furnace is essential for preparing nitrogen-doped porous carbon (SNCB) because it provides a sealed, oxygen-free environment and the precise thermal control needed to execute a complex two-stage heating profile. By maintaining a protective nitrogen atmosphere, the furnace allows for distinct pre-carbonization and activation phases, ensuring that chemical precursors react fully to create a stable, highly doped carbon framework.
Core Takeaway: The success of SNCB preparation hinges on the furnace’s ability to stabilize a specific "thermal field." This stability allows melamine and Metal-Organic Framework (MOF) precursors to undergo deep interaction—first reacting at lower temperatures and then activating at high heat—resulting in high nitrogen content and a developed hierarchical pore structure.
Precision Control of the Two-Stage Process
Executing the Thermal Ramping Strategy
The preparation of SNCB is not a single-step heating event; it requires a calculated two-stage approach. The tube furnace provides the programmable accuracy to hold the material first at a pre-carbonization temperature of 550 °C, followed by a controlled ramp to a high-temperature activation phase at 800 °C.
Ensuring Precursor Reactivity
This specific thermal profile is designed to maximize the interaction between the raw materials. The stable heat ensures that the nitrogen source (melamine) reacts completely with the carbon skeleton provided by the MOF precursor.
Solidifying the Carbon Framework
By strictly adhering to these temperature setpoints, the furnace facilitates deep pyrolysis. This removes volatile components at the correct rate, leaving behind a robust, chemically doped carbon structure rather than a collapsed or amorphous mass.
The Critical Role of Atmosphere Management
Preventing Material Oxidation
A tube furnace excels at maintaining a sealed, inert environment. A continuous flow of nitrogen gas is required to protect the organic precursors from combustion, which would occur instantly if exposed to oxygen at these processing temperatures.
Facilitating High-Temperature Activation
During the 800 °C stage, the inert atmosphere allows for thermal activation without burning away the carbon yield. This high-temperature phase is responsible for "etching" the material, creating the hierarchical pore structure that gives SNCB its high surface area.
Common Pitfalls in Thermal Processing
The Risk of Thermal Fluctuation
If the furnace cannot maintain a stable thermal field, the reaction between melamine and the MOF will be inconsistent. Inaccurate temperatures lead to uneven doping, where nitrogen is not uniformly integrated into the carbon lattice.
Consequences of Atmospheric Leaks
The sealing performance of the tube furnace is a critical failure point. Even minor oxygen ingress during the high-temperature activation phase (800 °C) will degrade the pore structure and drastically reduce the yield by converting the carbon into carbon dioxide.
Making the Right Choice for Your Goal
To maximize the quality of your SNCB material, focus on the specific capabilities of your thermal equipment.
- If your primary focus is High Nitrogen Doping: Prioritize a furnace with exceptional thermal stability at the lower 550 °C stage to ensure the melamine reacts fully before volatizing.
- If your primary focus is Hierarchical Pore Structure: Ensure your furnace supports precise high-temperature control at 800 °C and strictly regulates the nitrogen flow to facilitate optimal activation.
Ultimately, the tube furnace acts not just as a heater, but as a precise chemical reactor that dictates the final atomic architecture of your carbon material.
Summary Table:
| Pyrolysis Stage | Temperature | Primary Function | Key Outcome |
|---|---|---|---|
| Pre-carbonization | 550 °C | Interaction between melamine & MOF | Deep nitrogen doping into carbon lattice |
| Thermal Activation | 800 °C | Volatile removal & etching | Formation of hierarchical pore structure |
| Atmosphere Control | Room to 800 °C | Nitrogen flow (Inert) | Prevents oxidation and preserves carbon yield |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect hierarchical pore structure and nitrogen-doping levels requires more than just heat; it requires a stable, controlled thermal environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, and Vacuum systems—all customizable for your unique research or production needs.
Whether you are preparing SNCBs, specialized MOFs, or advanced ceramics, our CVD systems and lab high-temp furnaces provide the atmospheric integrity and programmable accuracy your work demands.
Ready to optimize your two-stage pyrolysis? Contact our experts today to find the perfect furnace solution for your lab.
Visual Guide
References
- Synergistic Cu-Pd Nanocatalysts on MOF-Derived N-Doped Carbon for Selective Hydrogenolysis of Lignin to Aromatic Monomers. DOI: 10.3390/catal15050455
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- Why is a High-Temperature Vacuum Tube Furnace required for the long-term homogenization of alloy ingots?
- What heat treatment processes can a 70mm tube furnace be used for? Essential Guide for Material Processing
- What role does a tube furnace play in converting nickel precursors? Master Thermal Reduction in Argon Atmospheres
- What process conditions are provided by a horizontal tube furnace for AuNPs@MOF catalysts? Precise Thermal Control
- What role does a horizontal tube furnace play in the carbonization of ionic liquid precursors? Master Thermal Control
- What is the purpose of using an industrial-grade vertical tube furnace in phosphorus recovery? High-Fidelity Simulation
- Why is a high-temperature tube furnace required for the synthesis of Fe-N-C catalysts? Key for Atomic Engineering
- What is the role of a tube furnace system in the growth of bilayer MoS2? Master CVD Synthesis with Precision Control



















