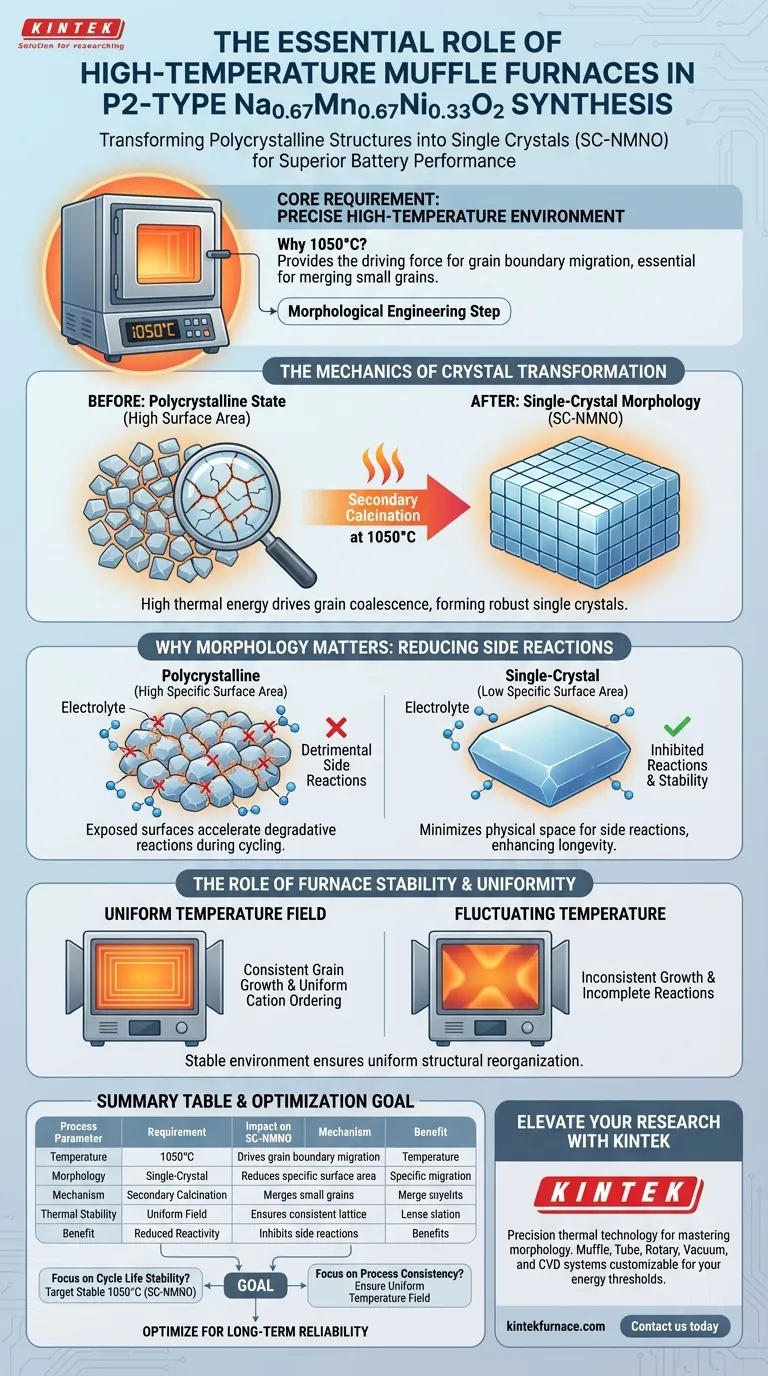
A high-temperature muffle furnace is required because it provides the precise thermal environment needed to drive grain growth and transform polycrystalline structures into single crystals. specifically, a stable temperature of 1050°C acts as the driving force for merging small grains, resulting in a robust material known as single-crystal P2-type Na0.67Mn0.67Ni0.33O2 (SC-NMNO).
Core Takeaway The secondary calcination process is not just about heating; it is a morphological engineering step. By utilizing high heat to fuse multiple small grains into a single large crystal, you significantly reduce the material's specific surface area, which is the primary mechanism for preventing degradative side reactions during battery cycling.
The Mechanics of Crystal Transformation
Driving Grain Boundary Migration
The primary function of the muffle furnace in this context is to provide a consistent 1050°C environment.
At this specific temperature, the thermal energy is sufficient to initiate and sustain grain boundary migration. This mechanism allows smaller grains to coalesce and merge, fundamentally altering the microstructure of the material.
Achieving Single-Crystal Morphology
The result of this migration is the transformation of the material from a polycrystalline state into large-sized single crystals (SC-NMNO).
Unlike polycrystalline materials, which are composed of many small, randomly oriented crystallites, a single crystal possesses a continuous and unbroken lattice structure. This transformation is impossible without the sustained high thermal energy provided by the furnace.
Why Morphology Matters for Performance
Reducing Specific Surface Area
The transition to a single-crystal morphology has a direct physical impact: it drastically reduces the specific surface area of the cathode material.
Polycrystalline materials inherently have a high surface-to-volume ratio due to the presence of many grain boundaries and exposed surfaces. By merging these grains, the total exposed surface area is minimized.
Inhibiting Interfacial Side Reactions
The reduction in surface area is the critical factor for battery longevity.
During battery cycling, the interface between the cathode and the electrolyte is where detrimental side reactions typically occur. By minimizing the exposed surface area through high-temperature calcination, you effectively limit the physical space available for these reactions, thereby stabilizing the material.
The Role of Furnace Stability
Providing a Stable Temperature Field
Beyond achieving the peak temperature, the muffle furnace must maintain a stable temperature field.
Fluctuations in temperature can lead to inconsistent grain growth or incomplete solid-state reactions. A stable environment ensures that the structural reorganization—where sodium, nickel, and manganese elements enter specific lattice sites—occurs uniformly throughout the batch.
Facilitating Solid-State Reactions
The heat acts as the driving force for the necessary solid-state reactions and crystallization.
While lower temperatures (e.g., 900-950°C) may allow for some structural reorganization and cation ordering, the specific goal of secondary calcination for SC-NMNO requires the higher energy threshold to fully realize the single-crystal form.
Understanding the Trade-offs
The Risk of Incomplete Calcination
If the furnace fails to maintain the required high temperature (1050°C), the grain growth process will be insufficient.
This results in a material that retains a polycrystalline nature with a higher specific surface area. While this might offer different electrochemical properties, it forfeits the stability benefits gained from inhibiting interfacial side reactions.
Balancing Crystallinity and Reactivity
There is a delicate balance between maximizing crystallinity and maintaining electrochemical activity.
In other catalytic contexts, excessive sintering (e.g., at 800°C for certain porous materials) can collapse pore structures and reduce active sites. However, for P2-type Na0.67Mn0.67Ni0.33O2, the "sintering" effect of merging grains is a desired outcome to enhance structural durability over surface reactivity.
Making the Right Choice for Your Goal
To optimize the synthesis of P2-type sodium-ion battery cathodes, align your thermal treatment with your specific performance objectives:
- If your primary focus is Cycle Life Stability: Target a stable 1050°C calcination to produce single crystals (SC-NMNO), which minimizes surface area and inhibits side reactions.
- If your primary focus is Process Consistency: Ensure your muffle furnace provides a uniform temperature field to prevent uneven cation mixing and ensure identical crystallinity across the entire sample batch.
Precise high-temperature regulation is the definitive tool for engineering the surface architecture required for long-term battery reliability.
Summary Table:
| Process Parameter | Requirement | Impact on SC-NMNO Material |
|---|---|---|
| Temperature | 1050°C | Provides driving force for grain boundary migration |
| Morphology | Single-Crystal | Reduces specific surface area and structural defects |
| Mechanism | Secondary Calcination | Merges small grains into large, robust single crystals |
| Thermal Stability | Uniform Field | Ensures consistent cation ordering and lattice structure |
| Benefit | Reduced Reactivity | Inhibits detrimental electrolyte-cathode side reactions |
Elevate Your Battery Material Research with KINTEK
Precision in secondary calcination is the difference between polycrystalline instability and single-crystal durability. KINTEK provides the advanced thermal technology needed to master the morphology of P2-type Na0.67Mn0.67Ni0.33O2.
Backed by expert R&D and world-class manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all customizable to meet your specific research or production energy thresholds. Whether you need a stable 1050°C environment or specialized atmospheres, KINTEK high-temperature furnaces deliver the uniform temperature fields required for superior material engineering.
Ready to optimize your synthesis process? Contact us today to find your custom furnace solution.
Visual Guide
References
- Venkat Pamidi, Maximilian Fichtner. Single-Crystal P2–Na<sub>0.67</sub>Mn<sub>0.67</sub>Ni<sub>0.33</sub>O<sub>2</sub> Cathode Material with Improved Cycling Stability for Sodium-Ion Batteries. DOI: 10.1021/acsami.3c15348
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How does muffle furnace temperature control affect high-activity bagasse ash? Master Your Calcination Precision
- Why is application important when selecting a muffle furnace? Ensure Optimal Performance for Your Lab
- How is a laboratory high-temperature muffle furnace utilized to achieve the specific crystalline structure of LaFeO3 catalysts?
- Why is an industrial muffle furnace required for preheating Fe-C-B-Cr-W alloys? Ensure Structural Integrity
- What is the range of a muffle furnace? Choosing the Right Temperature for Your Application
- What role does a laboratory muffle furnace play in the industrial analysis of plastic waste? Optimizing Pyrolysis Yield
- What safety features are enhanced in muffle furnaces? Discover Advanced Protection for Your Lab
- What potential hazards are associated with benchtop furnaces? Essential Safety Guide for Lab Users



















