High-purity Alumina (Al2O3) is chosen primarily for its exceptional thermal stability and mechanical integrity in extreme environments. Specifically, it is one of the few materials that can withstand insertion into liquid slag at 1600°C without immediate softening or structural failure, ensuring hydrogen is delivered effectively.
Success in hydrogen-based slag reduction relies on maintaining a stable injection path deep into the melt. High-purity Alumina provides the critical combination of a high melting point and structural rigidity to deliver hydrogen bubbles precisely where they are needed for maximum reaction efficiency.

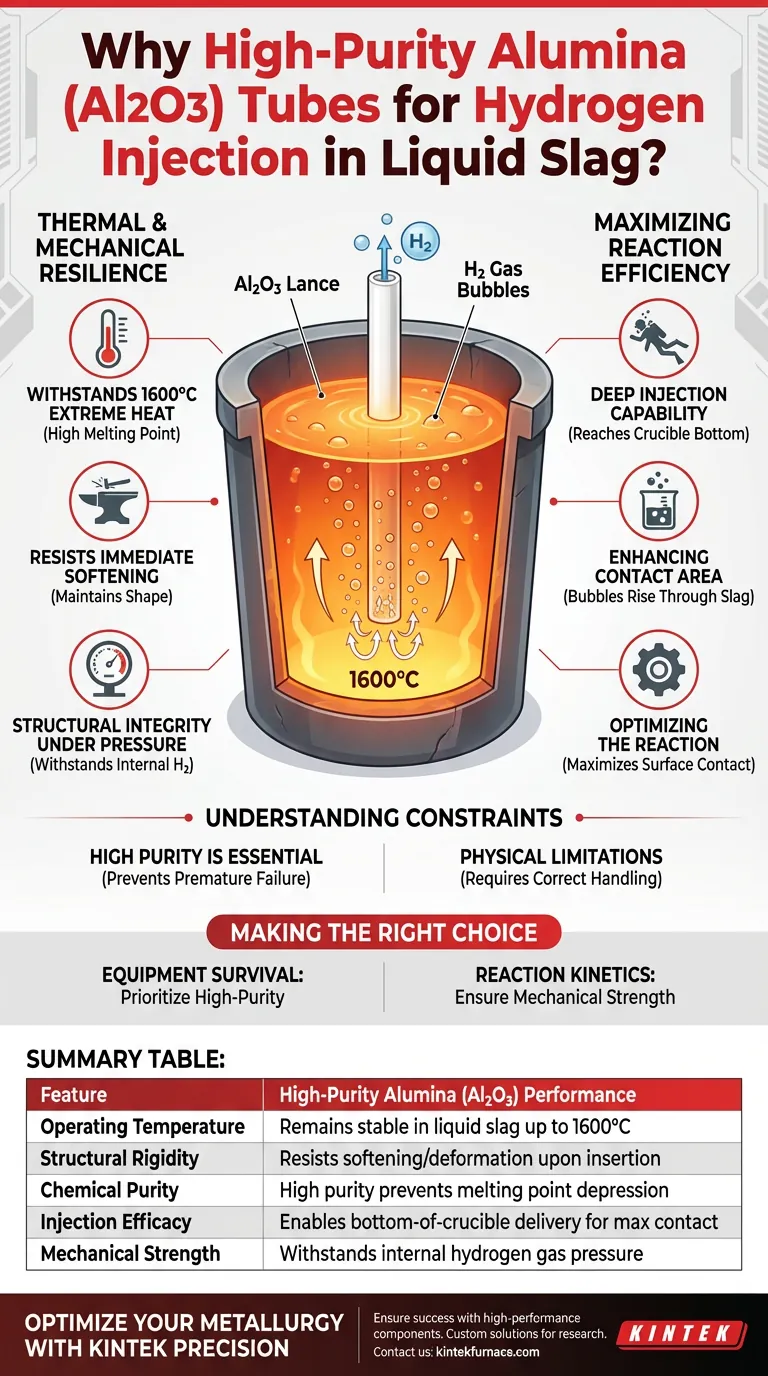
Thermal and Mechanical Resilience
Withstanding Extreme Heat
The operating environment for this process involves liquid slag at temperatures around 1600°C. High-purity Alumina is selected because it possesses a sufficiently high melting point to survive this environment.
Resisting Softening
Unlike lower-grade materials or metals that might deform instantly, Alumina maintains its shape. It does not suffer from immediate softening upon insertion. This rigidity is essential for the tube to function as a reliable lance.
Structural Integrity Under Pressure
The tube serves a dual purpose: it resists the heat outside and the pressure inside. It must maintain enough mechanical strength to withstand the internal pressure of the hydrogen gas being pumped through it without bursting or collapsing.
Maximizing Reaction Efficiency
Deep Injection Capability
The primary goal of the lance is to deliver gas to a specific location. Because the Alumina tube remains rigid, it can be inserted all the way to the bottom of the crucible.
Enhancing Contact Area
Delivering gas to the bottom is not arbitrary; it is a functional requirement. By releasing hydrogen at the lowest point, the lance creates bubbles that rise through the molten slag.
Optimizing the Reaction
These rising bubbles maximize the contact area between the reducing hydrogen gas and the iron oxides suspended in the slag. This maximized surface contact is critical for driving the reduction reaction efficiently.
Understanding the Constraints
The Necessity of High Purity
The specification of high-purity Alumina is not a suggestion; it is a requirement. Impurities in the ceramic matrix can significantly lower the melting point or structural strength, leading to premature failure at 1600°C.
Physical Limitations
While Alumina has "sufficient" strength, it is not indestructible. The material is selected specifically to bridge the gap between thermal resistance and the mechanical stress of insertion, but it must be handled correctly to avoid exceeding its physical limits during the process.
Making the Right Choice for Your Goal
To ensure your hydrogen injection process is successful, consider these key factors:
- If your primary focus is Equipment Survival: Prioritize high-purity Alumina grades to ensure the lance retains its rigidity and does not soften at 1600°C.
- If your primary focus is Reaction Kinetics: Ensure the lance has sufficient mechanical strength to reach the bottom of the crucible, guaranteeing the bubble formation necessary for oxide reduction.
Selecting the correct lance material is the foundational step that allows the chemistry of iron oxide reduction to occur efficiently.
Summary Table:
| Feature | High-Purity Alumina (Al2O3) Performance |
|---|---|
| Operating Temperature | Remains stable in liquid slag up to 1600°C |
| Structural Rigidity | Resists softening/deformation upon insertion |
| Chemical Purity | High purity prevents melting point depression |
| Injection Efficacy | Enables bottom-of-crucible delivery for max contact |
| Mechanical Strength | Withstands internal hydrogen gas pressure |
Optimize Your Metallurgy with KINTEK Precision
Ensure the success of your hydrogen-based slag reduction with high-performance components from KINTEK. Backed by expert R&D and world-class manufacturing, we provide high-purity Alumina tubes, Muffle, Tube, Rotary, Vacuum, and CVD systems designed to withstand the most demanding lab environments.
Whether you need standard specifications or a system fully customizable for your unique research needs, KINTEK delivers the thermal and mechanical resilience your process requires.
Ready to upgrade your high-temperature capabilities? Contact us today to discuss your project requirements!
Visual Guide
References
- M. A. Levchenko, Olena Volkova. Reduction of Liquid Steelmaking Slag Using Hydrogen Gas as a Reductant. DOI: 10.3390/met15090984
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Magnesium Extraction and Purification Condensing Tube Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
People Also Ask
- What is the significance of using high-purity quartz tubes in MoS2 growth? Ensure High-Purity Crystal Synthesis
- What role does a copper mold play in the formation of glass samples? Master Rapid Quenching & Amorphous Solidification
- What advantages do boron nitride (BN) crucibles offer for molten FUNaK salt? Ensure Purity & High-Temp Stability
- What is the function of high-purity refractory dies in slip-casting? Key Benefits for Precision Ceramic Molding
- What are the functions of Silica Quartz Tubes and quartz glass wool in RDF pyrolysis? Enhancing Experimental Accuracy
- What is the function of a vacuum drying oven for biochar FTIR analysis? Ensure High-Purity Sample Preparation
- How do alumina ceramic tubes compare to quartz ceramic tubes in terms of thermal properties? Choose the Right Tube for High-Temp Success
- How does a precision programmed cooling system influence the structural integrity of Al2O3-TiC composite materials?



















