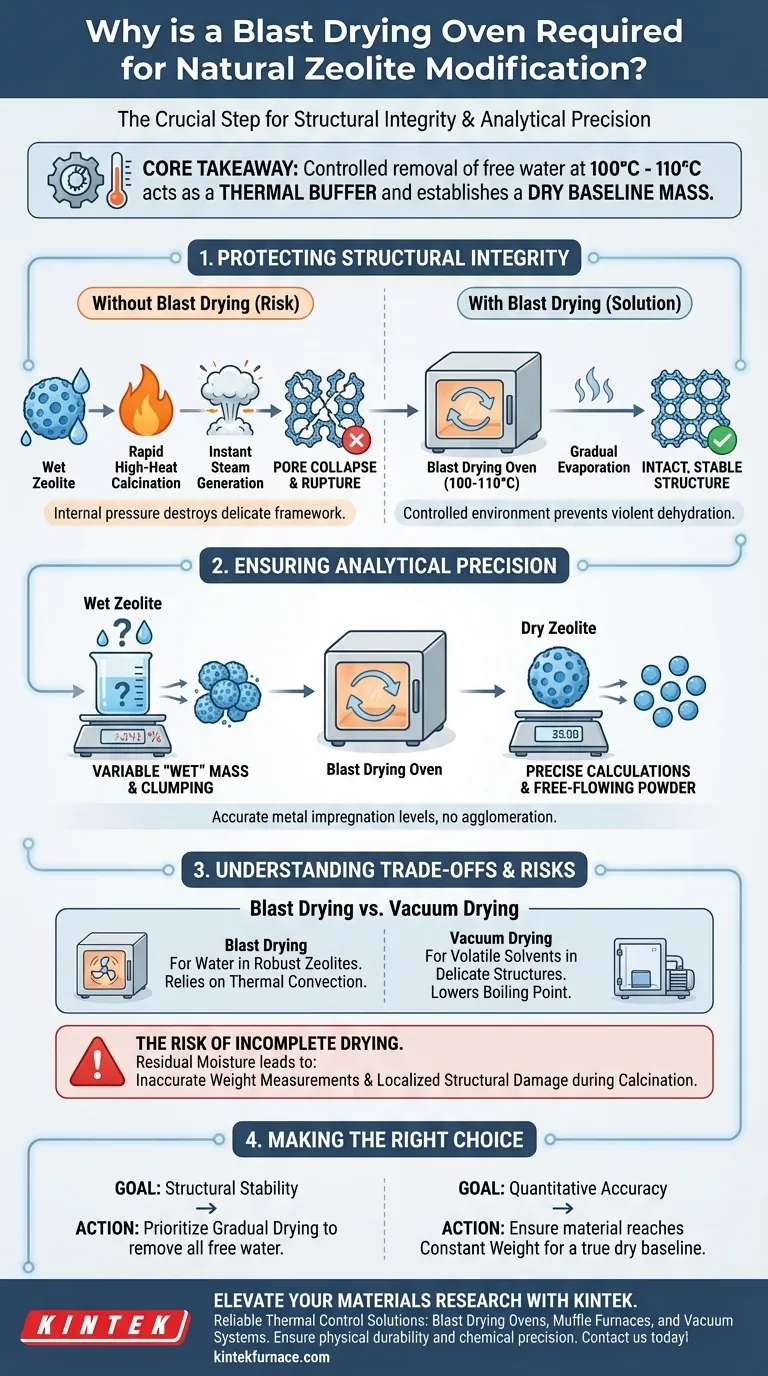
The blast drying oven is the primary instrument for the controlled removal of free water from natural zeolites during modification stages such as washing, impregnation, and desilication. Operating consistently between 100°C and 110°C, it serves as a critical stabilization step before high-temperature processing.
Core Takeaway The blast drying oven serves two essential functions: it acts as a thermal buffer to prevent structural collapse caused by rapid steam generation during calcination, and it establishes a standardized "dry mass" to ensure accurate calculations of chemical impregnation levels.
Protecting Structural Integrity
Preventing Pore Collapse
The most critical physical risk to zeolites during modification is the transition from a wet state to high-temperature calcination. If water-saturated zeolites are immediately exposed to extreme heat, the internal water converts to steam instantly.
This rapid phase change causes massive volume expansion within the material. Without a preliminary drying step, this internal pressure can rupture the zeolite's delicate porous framework, destroying the very structure you are trying to modify.
The Thermal Buffer
The blast drying oven mitigates this risk by providing a controlled environment at 100°C to 110°C. This temperature is sufficient to evaporate free water but low enough to do so gradually.
By removing the bulk of the water at this moderate stage, you ensure that the subsequent high-temperature calcination focuses on chemical activation rather than violent physical dehydration.
Ensuring Analytical Precision
Establishing a Dry Baseline Mass
To modify zeolites effectively, you must know the exact ratio of active metals or chemicals added to the support structure. However, "wet" zeolites contain an unknown and variable amount of water weight.
Drying the material establishes a dry baseline mass. This allows for precise calculations of metal impregnation levels, ensuring that the final composition matches your theoretical design.
Preventing Material Agglomeration
Moisture acts as a binding agent that can cause powders to clump together. While this is noted in sludge processing, the principle applies to zeolites as well.
Thorough drying ensures the material remains a free-flowing powder. This prevents clumping during handling and ensures uniform particle distribution during any subsequent grinding or mixing steps.
Understanding the Trade-offs
Blast Drying vs. Vacuum Drying
It is important to select the right drying method for the specific solvent involved. A blast drying oven relies on thermal convection and is ideal for removing water from robust natural zeolites.
However, for more delicate structures containing volatile organic solvents (like methanol in Metal-Organic Frameworks), a vacuum oven is often required. Vacuum drying lowers the boiling point, allowing solvent removal at lower temperatures (e.g., 40°C) to prevent framework collapse, which might occur even at blast-drying temperatures.
The Risk of Incomplete Drying
If the drying duration is insufficient, residual moisture will remain deep within the pores. Even a small amount of trapped water can compromise the accuracy of your weight measurements.
Furthermore, any remaining moisture can still lead to localized structural damage when the material is eventually moved to the calcination furnace.
Making the Right Choice for Your Goal
- If your primary focus is Structural Stability: Prioritize the blast drying step to remove all free water gradually, preventing steam-induced pore collapse during calcination.
- If your primary focus is Quantitative Accuracy: Ensure the material reaches a constant weight in the oven to establish a true dry baseline for calculating metal loading percentages.
By treating the drying phase as a critical quality control step rather than a simple pause, you ensure both the physical durability and chemical precision of your modified zeolites.
Summary Table:
| Feature | Blast Drying Oven Function | Importance in Zeolite Modification |
|---|---|---|
| Temperature Range | 100°C - 110°C | Controlled removal of free water without damaging pores |
| Structural Safety | Thermal Buffer | Prevents internal steam expansion and framework rupture |
| Analytical Accuracy | Dry Baseline Mass | Ensures precise calculation of metal/chemical loading |
| Material Quality | Anti-Agglomeration | Maintains free-flowing powder for uniform distribution |
| Process Flow | Pre-Calcination Step | Stabilizes material before high-temperature activation |
Elevate Your Materials Research with KINTEK
Precision in zeolite modification starts with reliable thermal control. KINTEK provides industry-leading Blast Drying Ovens, Muffle Furnaces, and Vacuum Systems designed to protect your material’s structural integrity and ensure analytical repeatability.
Backed by expert R&D and precision manufacturing, our lab solutions—including Tube, Rotary, and CVD systems—are fully customizable to meet your unique high-temperature processing needs.
Don't risk pore collapse or inconsistent data. Contact KINTEK today to find the perfect thermal solution for your laboratory!
Visual Guide
References
- Citronellal Acetylation Using Ni-Co Metal Impregnated Hierarchical Zeolite Catalysis and Its Potential as an Antibacterial, Antifungal and Antioxidants. DOI: 10.1051/e3sconf/202562202002
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1200℃ Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What conditions are required for grafting norbornene functional groups onto S-glass fiber surfaces? Expert Protocol
- Why is precision temperature control critical for MnBi2Te4 growth? Ensure Success in Flux Method Crystallization
- What role does X-ray diffraction (XRD) play in evaluating ZIF thermal treatment? Master Material Transformation
- Why is a multiple high-temperature tempering process necessary for high-cobalt steels? Achieve Peak Hardness & Stability
- What are the advantages of using an RTA system for CBTSe films? Precision Heating for Superior Thin Film Stoichiometry
- How does a sputtering system contribute to the preparation of electrodes? Enhance Bismuth Telluride Characterization
- What are the advantages of using multi-stage laboratory sintering furnaces? Ensure Defect-Free Powder Metallurgy
- How does a magnetic stirring hot plate contribute to sol-gel synthesis? Expert Guide to Precursor Thin Film Success



















