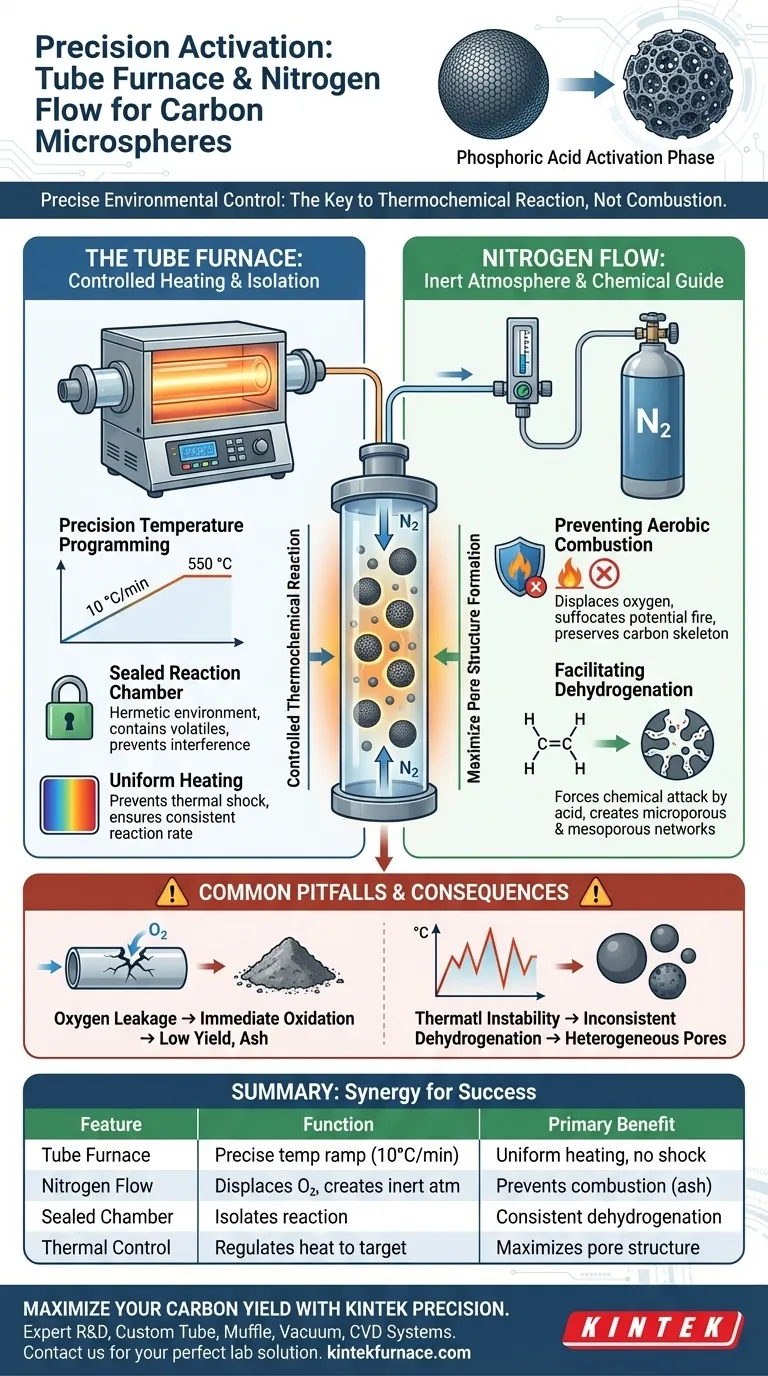
Precise environmental control is the primary operational requirement. A tube furnace is necessary to execute exact temperature ramps (typically 10 °C per minute) within a sealed chamber, while the nitrogen flow is critical to displace oxygen. Together, they ensure the phosphoric acid chemically activates the carbon framework rather than simply burning it away.
The synergy between the tube furnace and nitrogen flow transforms the heating process from destructive combustion into a controlled thermochemical reaction. This specific environment is required to facilitate dehydrogenation, preserving the carbon mass while maximizing the formation of microporous and mesoporous structures.
The Critical Role of the Tube Furnace
Precision Temperature Programming
The activation of carbon microspheres is not merely about reaching a high temperature; it is about how you get there. A tube furnace allows for programmable heating rates, such as a steady ramp of 10 °C per minute.
This controlled ascent ensures that the material is heated uniformly. It prevents thermal shock and allows the chemical reactions to proceed at a consistent rate up to the target temperature of 550 °C.
Creating a Sealed Reaction Chamber
A standard oven cannot provide the necessary isolation for this process. A tube furnace provides a hermetically sealed environment essential for chemical activation.
This isolation is the first step in managing the thermodynamics of the reaction. It contains the volatile components released during heating and ensures the external atmosphere does not interfere with the sample.
The Necessity of Nitrogen Flow
Preventing Aerobic Combustion
At the activation temperature of 550 °C, carbon is highly reactive with oxygen. Without a protective barrier, the carbon material would undergo aerobic combustion.
Nitrogen flow creates an inert atmosphere that envelops the microspheres. This effectively suffocates any potential fire, ensuring the carbon skeleton remains intact rather than turning into ash.
Facilitating the Dehydrogenation Reaction
The goal of using phosphoric acid is to induce a specific chemical change called dehydrogenation. This reaction removes hydrogen from the carbon framework to open up pore structures.
The nitrogen environment ensures that this is the dominant reaction. By excluding oxygen, the system forces the phosphoric acid to attack the carbon chemically, creating extensive microporous and mesoporous networks.
Common Pitfalls to Avoid
The Consequence of Oxygen Leakage
Even a minor failure in the nitrogen seal can be catastrophic. If oxygen enters the tube during the high-temperature phase, the protective inert atmosphere is compromised.
This results in the immediate oxidation of the carbon. You will likely end up with a significantly lower yield and a sample composed mostly of useless ash rather than porous microspheres.
The Impact of Thermal Instability
Attempting this process without the precise control of a tube furnace often leads to uneven activation. If the temperature fluctuates or ramps too quickly, the dehydrogenation process becomes inconsistent.
This inconsistency leads to heterogeneous pore structures. The final material will lack the high specific surface area required for high-performance applications.
Making the Right Choice for Your Goal
To ensure you achieve high-quality carbon microspheres, apply these principles based on your specific objectives:
- If your primary focus is maximizing surface area: Ensure your nitrogen flow is continuous and robust to prevent any oxidation that would close off micropores.
- If your primary focus is structural consistency: Rely on the tube furnace’s strict programming to maintain a linear 10 °C/min ramp, avoiding thermal spikes that damage the carbon framework.
Ultimately, these tools are required not just to heat the material, but to orchestrate a precise chemical attack that sculpts the carbon on a microscopic level.
Summary Table:
| Feature | Function in Phosphoric Acid Activation | Primary Benefit |
|---|---|---|
| Tube Furnace | Precise temperature programming (10 °C/min) | Uniform heating & prevents thermal shock |
| Nitrogen Flow | Displaces oxygen to create an inert atmosphere | Prevents aerobic combustion (ash formation) |
| Sealed Chamber | Isolates the thermochemical reaction | Ensures consistent dehydrogenation |
| Thermal Control | Regulates heat up to target (e.g., 550 °C) | Maximizes microporous & mesoporous structure |
Maximize Your Carbon Yield with KINTEK Precision
Don't let oxygen leaks or thermal instability ruin your activation process. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Tube, Muffle, Vacuum, and CVD systems designed to handle the most rigorous lab requirements. Whether you need precise 10 °C/min ramping or a hermetically sealed environment for inert gas flow, our systems are fully customizable to your unique research needs.
Ready to elevate your material science? Contact us today to find the perfect furnace solution for your lab!
Visual Guide
References
- Saeed Alhawtali, Chun‐Yang Yin. Date Palm Leaflet-Derived Carbon Microspheres Activated Using Phosphoric Acid for Efficient Lead (II) Adsorption. DOI: 10.3390/c10010026
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What is the purpose of a Split Tube Furnace (Single Zone)? Ideal for Easy Access and Uniform Heating
- What environmental conditions must a high-temperature tube furnace provide for MAX phase sintering? Expert Guidelines
- What thermal processes can tube furnaces perform? Achieve Precise High-Temperature Control for Your Lab
- What are the differences between solid and split tube furnaces? Choose the Right Furnace for Your Lab
- Why is a stable argon atmosphere necessary when using a tube furnace for GH4099 alloy? Ensure Material Integrity
- Why is pre-oxidation treatment of the substrate in a tube furnace necessary? Ensure Strong Ti(Nb)-Si-C Coating Adhesion
- Why is a High-Temperature Vacuum Tube Furnace required for the long-term homogenization of alloy ingots?
- What is the role of a three-zone tube furnace in HPHT nanodiamond pretreatment? Unlock Precise Surface Activation



















