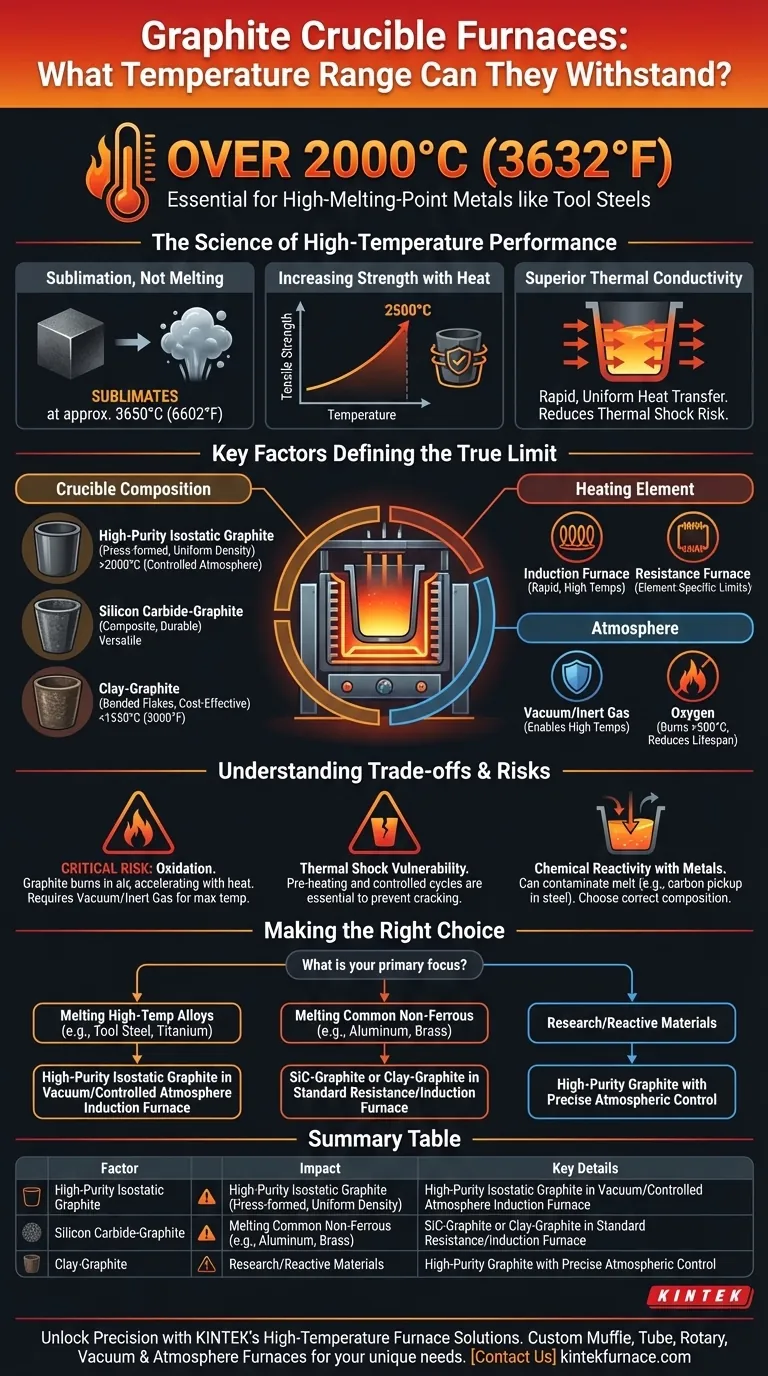
In short, a high-purity graphite crucible furnace can withstand temperatures exceeding 2000°C (3632°F). This extreme tolerance is why they are essential for smelting alloy tool steels and other high-melting-point metals. However, the crucible's material composition and the furnace's design dictate the true operational limits.
While graphite's physical properties allow for temperatures over 3000°C in a vacuum, a furnace's practical, reliable operating temperature is a system-level property. It is defined by the specific grade of the graphite crucible, the limits of the heating elements, and the presence of oxygen.

The Science Behind Graphite's High-Temperature Performance
Graphite is not simply "heat resistant"; its atomic structure gives it unique properties that make it ideal for high-temperature metallurgical work. Understanding these principles is key to using it effectively.
Sublimation, Not Melting
Under normal atmospheric pressure, graphite does not have a melting point. Instead, it sublimates—turning directly from a solid to a gas—at approximately 3650°C (6602°F). This incredibly high sublimation point establishes the theoretical temperature ceiling.
Increasing Strength with Heat
Unlike metals that weaken and soften as they approach their melting point, graphite exhibits a rare characteristic: its tensile strength increases with temperature. It grows stronger up to around 2500°C (4532°F), providing exceptional structural integrity when it's needed most.
Superior Thermal Conductivity
Graphite is an excellent thermal conductor. This allows for rapid and uniform heat transfer from the furnace elements through the crucible and into the metal charge. This efficiency reduces melting times and minimizes the risk of thermal shock—cracking caused by uneven temperature changes.
Key Factors Defining a Furnace's True Temperature Limit
The "over 2000°C" figure applies only to specific setups. In practice, the limit is determined by a combination of components.
The Crucible's Composition
Not all "graphite" crucibles are the same. The specific material dictates performance and cost.
-
High-Purity Isostatic Graphite: This is the highest-grade material, pressed to achieve uniform density. It is required for the most demanding applications, such as semiconductor manufacturing or melting reactive metals, and can operate in controlled atmospheres well above 2000°C.
-
Silicon Carbide-Graphite: A composite material that blends graphite with silicon carbide. It offers superior durability, mechanical strength, and oxidation resistance compared to clay-graphite. It is a versatile choice for melting both ferrous and non-ferrous metals.
-
Clay-Graphite: Graphite flakes are bonded with clay. This is a common and cost-effective choice for lower-temperature, non-ferrous metals like aluminum, brass, and bronze, with typical operating limits well below 1650°C (3000°F).
The Heating Element's Role
The crucible can only get as hot as the furnace's heating system allows. An induction furnace uses electromagnetic fields to heat the crucible directly and can achieve very high temperatures rapidly. A resistance furnace relies on heating elements (like silicon carbide or molybdenum disilicide) which have their own maximum service temperatures that may be lower than the crucible's limit.
Understanding the Trade-offs and Risks
High-temperature operation introduces significant challenges that must be managed to ensure safety, crucible longevity, and melt quality.
The Critical Risk of Oxidation
This is the single most important real-world limitation. Graphite will react with oxygen (burn) at high temperatures, beginning around 500°C (932°F). The rate of this oxidation accelerates dramatically as temperatures rise.
Operating a graphite crucible in open air significantly reduces its maximum practical temperature and drastically shortens its lifespan. The highest temperature ratings are only achievable in a vacuum or an inert gas atmosphere (like argon) to protect the crucible from being consumed.
Thermal Shock Vulnerability
Despite excellent thermal conductivity, a crucible can still crack. Pre-heating the crucible before charging it with cold metal and ensuring controlled heating and cooling cycles are critical operational procedures to prevent catastrophic failure from thermal shock.
Chemical Reactivity with Metals
Graphite is not entirely inert. It can react with certain molten metals. For example, molten iron will absorb carbon from a graphite crucible, which can alter the final chemistry of the steel and slowly degrade the crucible wall. Selecting the right crucible composition for the specific metal is essential to prevent contamination.
Making the Right Choice for Your Application
Selecting the correct system requires matching the technology to your specific goal. Look beyond the maximum temperature and consider the entire operational context.
- If your primary focus is melting high-temperature alloys like tool steel or titanium: You require a high-purity isostatic graphite crucible within a vacuum or controlled-atmosphere induction furnace.
- If your primary focus is melting common non-ferrous metals like aluminum or brass: A silicon carbide-graphite or clay-graphite crucible in a standard resistance or induction furnace is the most durable and cost-effective solution.
- If your primary focus is research or melting highly pure, reactive materials: Prioritize a high-purity graphite system with precise atmospheric control to prevent both crucible oxidation and melt contamination.
Choosing the right furnace is about understanding that it is a complete system, where the crucible is just one critical part of achieving your specific metallurgical goal.
Summary Table:
| Factor | Impact on Temperature Limit | Key Details |
|---|---|---|
| Crucible Material | Determines max temperature | High-purity graphite: >2000°C; Silicon carbide-graphite: versatile; Clay-graphite: <1650°C |
| Heating Element | Limits achievable heat | Induction furnaces: rapid high temps; Resistance furnaces: element-specific limits |
| Atmosphere | Prevents oxidation | Vacuum/inert gas enables high temps; Air exposure reduces limit and lifespan |
| Application | Guides material choice | Tool steel/titanium: high-purity; Aluminum/brass: cost-effective composites |
Unlock Precision with KINTEK's High-Temperature Furnace Solutions
Are you working with high-melting-point metals like tool steels or reactive materials? KINTEK leverages exceptional R&D and in-house manufacturing to provide advanced furnace systems tailored to your needs. Our product line includes Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems, all backed by strong deep customization capabilities to meet your unique experimental requirements.
Contact us today to discuss how our solutions can enhance your lab's efficiency and safety—let's achieve your metallurgical goals together!
Visual Guide
Related Products
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
People Also Ask
- What are the advantages of individually temperature-controlled zones in multi-zone furnaces? Unlock Precision Thermal Gradients
- How does a multi-zone tube furnace achieve precise temperature gradient control? Master MoS2 Isotope Monolayer Synthesis
- What steps are involved in the installation of a multi zone tube furnace? Ensure Precision and Safety for Your Lab
- How are multi zone tube furnaces used in ceramics, metallurgy and glass research? Unlock Precise Thermal Control for Advanced Materials
- How do multi zone tube furnaces improve laboratory efficiency? Boost Throughput with Parallel Processing



















