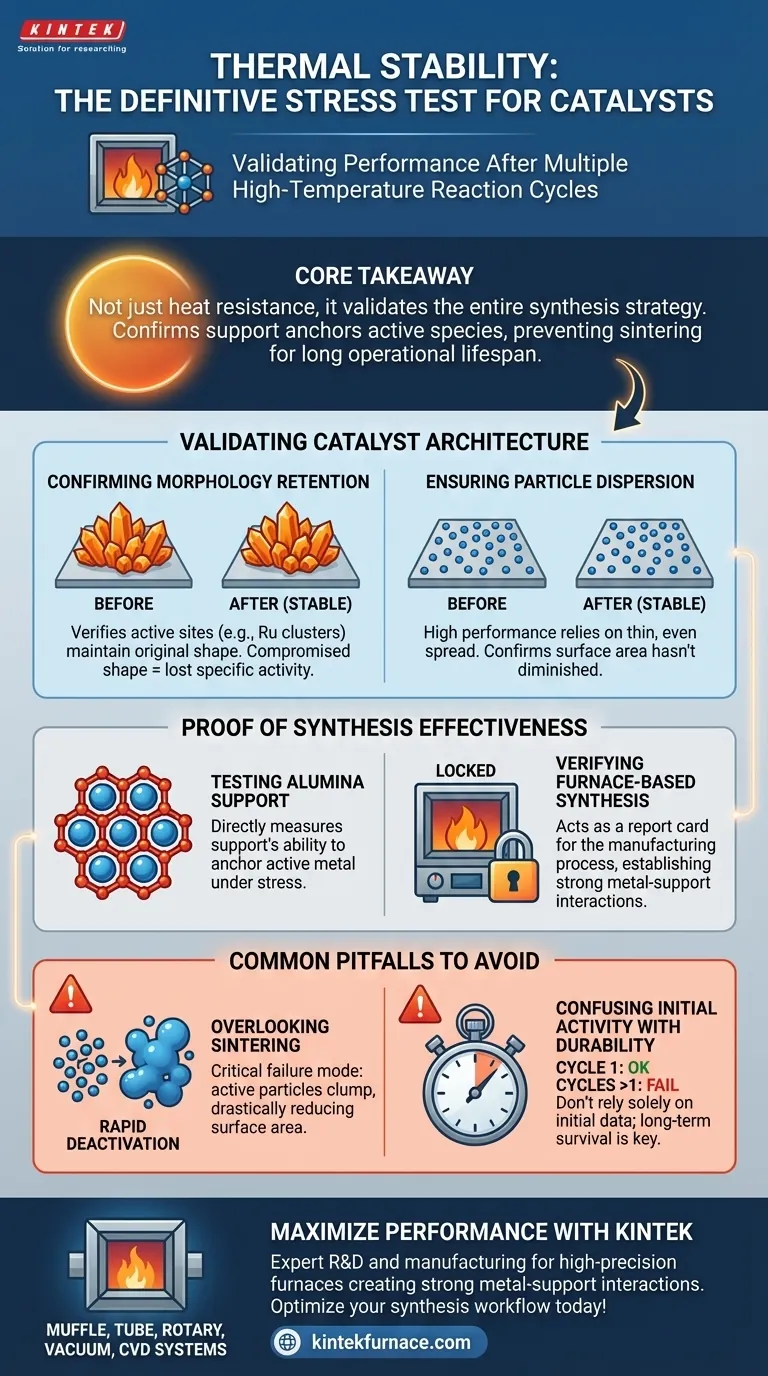
Thermal stability serves as the definitive stress test for any catalyst synthesized in a high-temperature environment. It acts as the primary metric to determine if the active sites, such as ruthenium clusters, retain their specific shape and distribution after surviving the harsh conditions of high-pressure reaction cycles.
Core Takeaway Thermal stability evaluation is not just about heat resistance; it validates the entire synthesis strategy. It confirms that the support structure effectively anchors active species to prevent sintering, ensuring the catalyst maintains its functionality over a long operational lifespan.

Validating Catalyst Architecture
Confirming Morphology Retention
The central role of this evaluation is to verify that the active sites have not physically degraded.
After exposure to reaction conditions, the catalyst is inspected to ensure the active clusters maintain their original morphology. If the shape changes, the specific chemical activity of the catalyst is compromised.
Ensuring Particle Dispersion
High performance depends on the active species being spread thinly and evenly across the support.
Thermal stability tests confirm that these particles have remained dispersed rather than migrating across the surface. This proves that the catalyst surface area available for reaction has not diminished during use.
Proof of Synthesis Effectiveness
Testing the Alumina Support
The evaluation directly measures the effectiveness of the support material, specifically the alumina structure.
It determines if the support is robust enough to hold the active metal in place under stress. A stable result indicates the support is successfully preventing the movement of metal clusters.
verifying Furnace-Based Synthesis
This assessment acts as a report card for the manufacturing process itself.
It confirms that the furnace-based synthesis method successfully established strong interactions between the metal and the support. If the catalyst remains stable, the high-temperature synthesis successfully "locked" the structure in place.
Common Pitfalls to Avoid
Overlooking Sintering
The most critical failure mode in these evaluations is sintering, where active particles clump together.
If an evaluation focuses only on chemical output without checking for sintering, you may miss physical degradation. This clumping drastically reduces the active surface area and leads to eventual failure.
Confusing Initial Activity with Durability
A catalyst may perform well in the first cycle but fail structurally shortly after.
Relying solely on initial reaction data is a mistake; thermal stability data is required to prove the catalyst can survive extended periods of use without deactivation.
Assessing Your Catalyst Needs
To ensure you are selecting or designing the right catalyst for your specific constraints, consider the following:
- If your primary focus is Long-Term Reliability: Prioritize catalysts where evaluation confirms zero significant changes in particle dispersion after multiple high-pressure cycles.
- If your primary focus is Validating Manufacturing: Use thermal stability data to confirm that your furnace synthesis temperatures are creating sufficient metal-support interactions to prevent sintering.
Ultimately, thermal stability is the only metric that guarantees your catalyst is robust enough to turn a theoretical design into a practical, long-lasting industrial solution.
Summary Table:
| Evaluation Metric | Role in Catalyst Performance | Impact of Failure |
|---|---|---|
| Morphology Retention | Maintains specific shape of active sites (e.g., Ru clusters) | Loss of specific chemical activity |
| Particle Dispersion | Ensures active species remain spread across support | Reduced surface area and reaction rates |
| Support Robustness | Anchors metal clusters via alumina structure | Particle migration and structural collapse |
| Sintering Resistance | Prevents clumping of active particles | Rapid deactivation and physical degradation |
Maximize Your Catalyst Performance with KINTEK
Is your research or industrial application demanding exceptional catalyst durability? At KINTEK, we understand that thermal stability starts with the synthesis process. Backed by expert R&D and manufacturing, we offer high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems designed to create the strong metal-support interactions your catalysts need to survive harsh reaction cycles.
Whether you require a standard solution or a customized high-temp furnace for unique research needs, our equipment ensures the morphology and dispersion of your active sites are locked in for long-term reliability. Contact us today to optimize your synthesis workflow!
Visual Guide
References
- DeSheng Su, Liang Chen. Efficient amine-assisted CO2 hydrogenation to methanol co-catalyzed by metallic and oxidized sites within ruthenium clusters. DOI: 10.1038/s41467-025-55837-7
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How do precision electric drying ovens control the precipitation of strengthening phases in recycled aluminum alloys?
- How does an electric furnace ensure accurate gasification? Master Isothermal and Dynamic Thermal Control
- What is the technical value of using a vacuum drying oven? Master Platinum Catalyst Precision and Activity
- What environmental conditions are critical for SiOC ceramicization? Master Precise Oxidation & Thermal Control
- What is made in a dental lab? Discover the Custom Prosthetics for Your Smile
- Why is a heating device required when evaluating HEAs? Unlocking High-Temperature Material Performance
- What is the role of high-pressure inert gases in the HPB process? Mastering CZT Crystal Stoichiometry
- Why is a stainless steel high-pressure autoclave essential for starch hydrogenation? Unlock Peak Reaction Efficiency



















