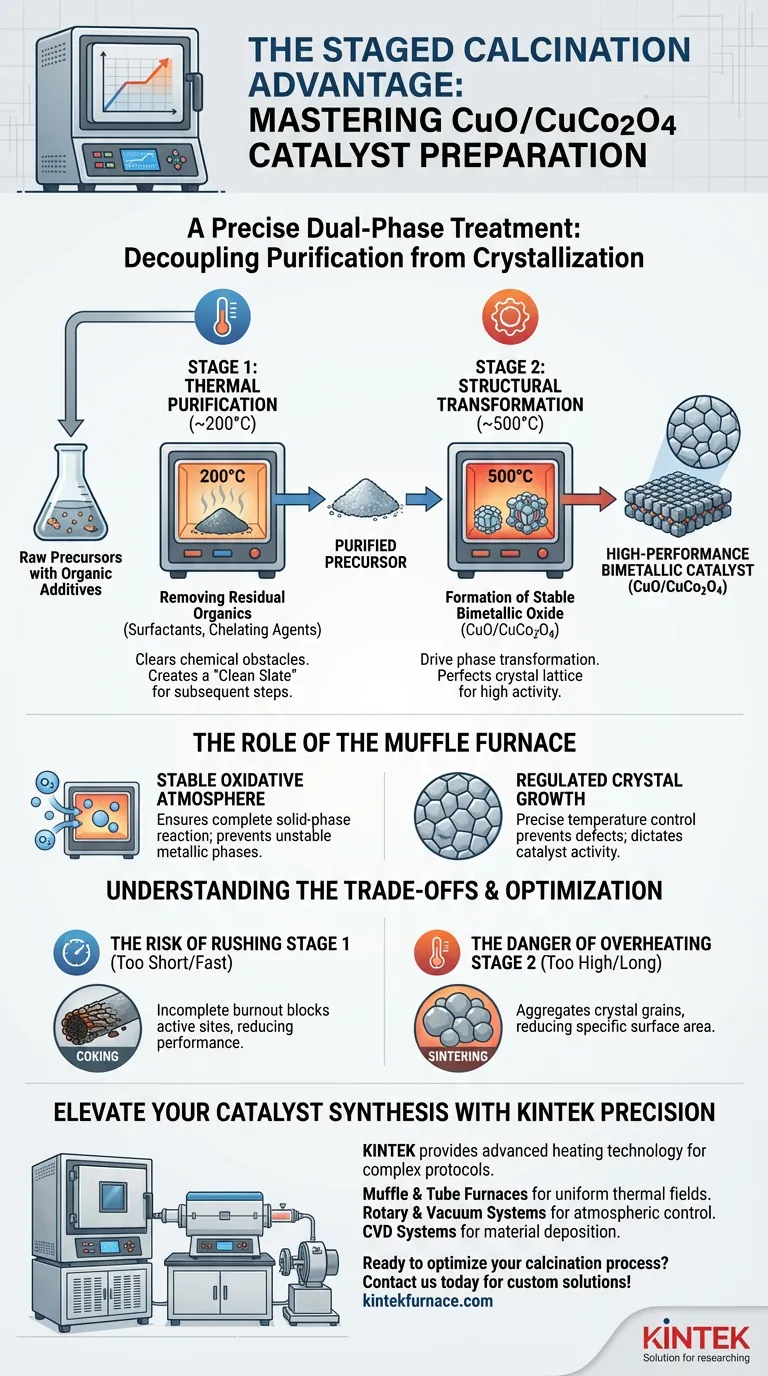
The staged calcination process acts as a precise, dual-phase treatment responsible for evolving raw precursors into a high-performance bimetallic catalyst. Utilizing the controlled environment of a muffle furnace, this method executes a specific temperature program—typically initiating at a lower tier (around 200°C) before escalating to a higher tier (around 500°C). This stepwise approach ensures that chemical impurities are eliminated before the critical crystal lattice forms, directly determining the final catalytic efficiency.
Core Takeaway: This process decouples purification from crystallization. By removing organic barriers at lower temperatures first, the system creates a "clean slate" that allows the subsequent high-temperature stage to form a structurally perfect, highly active bimetallic oxide composite without interference.

The Mechanics of the Two-Stage Process
The preparation of CuO/CuCo2O4 catalysts relies on a distinct separation of duties between two temperature zones. This "staged" approach prevents the chaotic reactions that can occur during rapid, single-step heating.
Stage 1: Thermal Purification (~200°C)
The primary objective of the initial low-temperature stage is cleaning the precursor.
During synthesis, precursors are often mixed with organic substances, such as surfactants or chelating agents, to control particle size or dispersion.
Holding the material at approximately 200°C steadily removes these residual organics through decomposition. This step clears chemical obstacles, ensuring that no carbonaceous debris remains to hinder the interaction of metal atoms in the next stage.
Stage 2: Structural Transformation (~500°C)
Once the material is purified, the furnace ramps up to the high-temperature stage (typically 500°C) to drive phase transformation.
At this thermal plateau, the metal precursors undergo a complete conversion into stable metal oxides.
This is where the bimetallic nature of the catalyst is defined. The heat induces the formation of the CuO/CuCo2O4 composite, perfecting the crystal structure. This distinct crystalline arrangement is the source of the material's high catalytic efficiency.
The Role of the Muffle Furnace Environment
The success of staged calcination depends heavily on the specific capabilities of the muffle furnace itself.
Providing a Stable Oxidative Atmosphere
For metal precursors to convert into active oxides (like CuO and CuCo2O4), they require a consistent supply of oxygen.
The muffle furnace maintains a stable oxidative environment throughout the heating ramp. This ensures that the solid-phase reactions between metal elements are complete, preventing the formation of incomplete or unstable metallic phases.
Regulating Crystal Growth
Catalytic activity is often dictated by the size and shape of the crystal grains.
The precise temperature control of a muffle furnace creates a uniform thermal field. This uniformity allows for regulated grain growth, preventing the structural defects that can occur in uneven heating environments.
Understanding the Trade-offs
While staged calcination is superior to single-step heating for complex catalysts, it requires careful optimization.
The Risk of "Rushing" Stage 1
If the low-temperature dwell time is too short or the ramp rate too fast, organic ligands may not burn off completely.
This results in carbon residues effectively "coking" the catalyst before it is even used, blocking active sites and significantly reducing performance.
The Danger of Overheating Stage 2
While high heat is necessary for crystallization, excessive temperature or duration can lead to sintering.
If the material is held at high temperatures for too long, the fine crystal grains may aggregate into larger clumps. This drastically reduces the specific surface area, lowering the number of active sites available for catalytic reactions.
Making the Right Choice for Your Goal
To optimize your CuO/CuCo2O4 catalyst preparation, align your furnace programming with your specific material requirements:
- If your primary focus is Maximizing Active Sites: Prioritize a slow ramp rate and sufficient hold time at the 200°C stage to ensure every trace of surfactant is removed without collapsing the pores.
- If your primary focus is Structural Stability: Ensure the 500°C stage is maintained long enough to fully crystallize the bimetallic oxides, but monitor closely to prevent the onset of thermal sintering.
Success lies in respecting the hierarchy of heat: purify first, then crystallize.
Summary Table:
| Stage | Temperature | Primary Function | Key Outcome |
|---|---|---|---|
| Stage 1 | ~200°C | Thermal Purification | Removal of organic impurities and surfactants |
| Stage 2 | ~500°C | Structural Transformation | Conversion to stable bimetallic oxide crystal structure |
| Atmosphere | Ambient/Oxygen | Oxidation | Ensures complete solid-phase reaction of metal elements |
| Control | Precise Ramp/Soak | Uniform Thermal Field | Prevents sintering and regulates crystal grain size |
Elevate Your Catalyst Synthesis with KINTEK Precision
Precise thermal processing is the difference between a contaminated precursor and a high-performance bimetallic catalyst. KINTEK provides the advanced heating technology required to master complex protocols like staged calcination.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of lab solutions, including:
- Muffle & Tube Furnaces: Delivering the uniform thermal fields essential for stable phase transformation.
- Rotary & Vacuum Systems: Optimized for specialized atmospheric control.
- CVD Systems: For advanced material deposition and synthesis.
All our high-temperature systems are fully customizable to meet your unique research or production needs. Ensure structural perfection in your materials with KINTEK’s industry-leading thermal accuracy.
Ready to optimize your calcination process? Contact us today to find your custom furnace solution!
Visual Guide
References
- Jin Li, Hao Li. Advancing Electrochemical Nitrate Reduction: Overcoming Rate‐Limiting Bottlenecks with Copper/Cobalt Catalysts. DOI: 10.1002/adfm.202513717
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the function of a box-type resistance furnace in Ni/C composite heat treatment? Expert Strengthening Guide
- How often should a muffle furnace be maintained? Optimize Performance with Proactive Care
- What is the primary objective of using a box annealing furnace for 3D ZnO nanostructures? Engineer Grain Growth
- What is the role of a high-temperature muffle furnace in glucose sensor prep? Optimize Metal Oxide Phase Transitions
- What is the function of a box-type resistance furnace in GFRP studies? Mastering High-Temperature Material Simulation
- How is a muffle furnace utilized in the preparation of carbon materials derived from L-valine? Master Carbonization
- What is sintering, and how is a muffle furnace used in this process? Unlock Precision in Material Bonding
- What temperature range can muffle furnaces reach? Find Your Ideal Lab Furnace Temperature



















