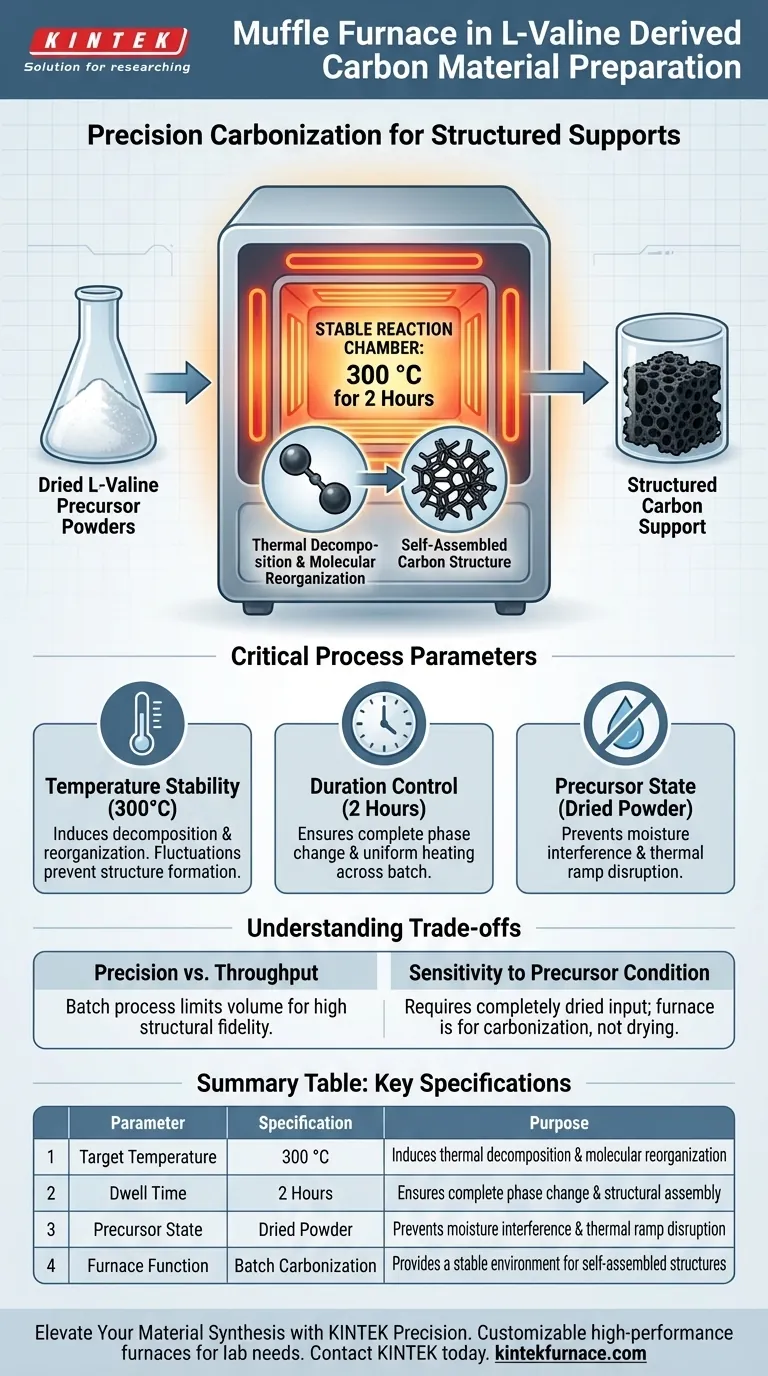
In the preparation of L-valine derived carbon materials, a muffle furnace serves as the critical reaction chamber for controlled carbonization. It is specifically utilized to subject dried L-valine precursor powders to a stable temperature of 300 °C for a duration of 2 hours. This precise thermal treatment drives the thermal decomposition of the amino acid molecules, converting them into a powdered carbon support with a specific, self-assembled structure.
The muffle furnace is not merely a heating element; it acts as a stabilizing vessel that allows for the uniform molecular reorganization of L-valine into structured carbon without uncontrolled combustion.

The Mechanics of Transformation
Thermal Decomposition
The primary function of the muffle furnace in this context is to induce thermal decomposition.
By raising the temperature to 300 °C, the furnace provides the energy required to break the organic bonds within the L-valine amino acid.
This process strips away volatile components, leaving behind a carbon-rich skeleton.
Molecular Reorganization
Beyond simple charring, the furnace environment facilitates a specific structural change.
The supplementary metallurgical context suggests that these high-temperature environments enable changes at a molecular level.
In the case of L-valine, the specific 2-hour hold time allows the material to form a self-assembled structure, resulting in a functional powdered carbon support rather than amorphous ash.
Critical Process Parameters
Temperature Stability
Success depends entirely on maintaining a fixed environment.
The furnace must hold the temperature specifically at 300 °C.
Fluctuations in this temperature could lead to incomplete carbonization or the destruction of the desired self-assembled architecture.
Duration Control
The reaction kinetics for this specific transformation require time to stabilize.
The protocol demands a strictly monitored period of 2 hours.
This duration ensures the heat penetrates the dried powder evenly, ensuring the entire batch undergoes the necessary phase change.
Understanding the Trade-offs
Precision vs. Throughput
Muffle furnaces generally operate as batch processing units.
While they offer excellent atmospheric control and temperature stability, they limit the volume of L-valine that can be processed continuously.
You must accept lower throughput to achieve the high structural fidelity required for this carbon material.
Sensitivity to Precursor Condition
The process relies on the input being dried L-valine precursor powders.
The muffle furnace is designed for carbonization, not initial drying.
Introducing moisture into the furnace at 300 °C can alter the thermal ramp and disrupt the formation of the self-assembled structure.
Making the Right Choice for Your Goal
To ensure the successful synthesis of carbon materials from L-valine, you must adhere to strict parameter controls.
- If your primary focus is Structural Integrity: strictly calibrate the furnace to 300 °C, as deviations will prevent the formation of the specific self-assembled support structure.
- If your primary focus is Material Uniformity: ensure the precursor powder is completely dried prior to insertion to guarantee an even thermal decomposition across the 2-hour cycle.
Mastering the use of the muffle furnace in this application requires viewing it as an instrument of molecular assembly, not just a heat source.
Summary Table:
| Parameter | Specification | Purpose |
|---|---|---|
| Target Temperature | 300 °C | Induces thermal decomposition and molecular reorganization |
| Dwell Time | 2 Hours | Ensures complete phase change and structural assembly |
| Precursor State | Dried Powder | Prevents moisture interference and thermal ramp disruption |
| Furnace Function | Batch Carbonization | Provides a stable environment for self-assembled structures |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the delicate molecular reorganization of L-valine into advanced carbon supports requires absolute thermal stability. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory needs. Whether you are scaling up batch processing or refining carbonization protocols, our furnaces provide the accuracy your research demands.
Ready to optimize your carbon material preparation? Contact KINTEK today for a customized furnace solution!
Visual Guide
References
- Parameswari R. Nithiasri, B. Karthikeyan. Novel self-assembled valine-derived carbon-supported Ag@ZnO optical materials for enhanced photodegradation and anti-bacterial activity. DOI: 10.1039/d5na00427f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the function of a high-temperature muffle furnace? Master Polycrystalline MgSiO3 and Mg2SiO4 Synthesis
- How does a laboratory muffle furnace facilitate the activation of ZMQ-1 zeolite? Unlock 28-Ring Pore Channels
- What role does a muffle furnace play in Al2O3/PTFE composites? Optimize Sintering for Superior Performance
- What is a muffle furnace? Achieve Pure, Controlled High-Temp Processing
- How is the furnace door of a box type resistance furnace secured and operated? Ensure Safety and Efficiency in Your Lab
- What role do muffle furnaces play in semiconductor material processing? Essential for Precise Annealing and Dopant Activation
- How does a high-temperature box muffle furnace convert mussel shells to calcium oxide? Expert Calcination Guide
- Why is a high-temperature muffle furnace required for 1000 Celsius aging treatment? Evaluate CeZrPAl Durability



















