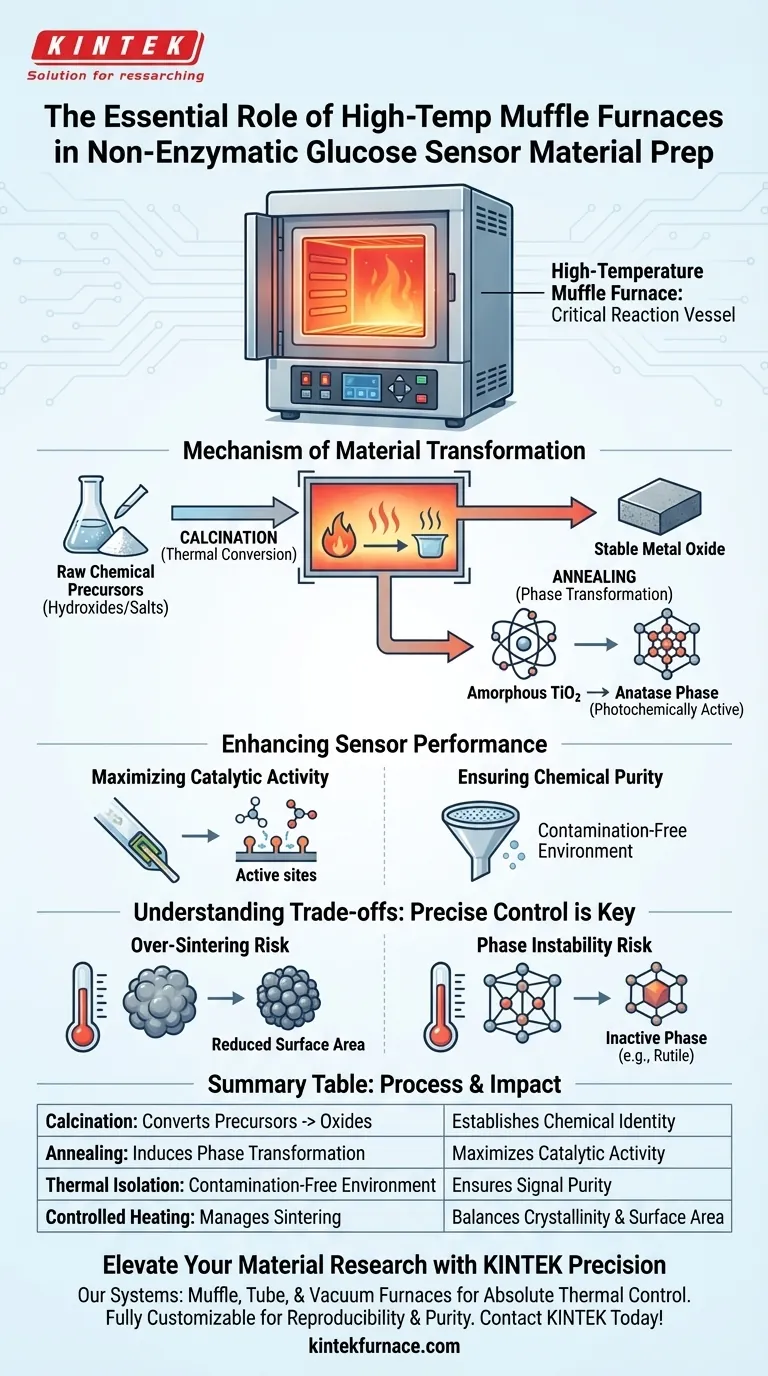
The high-temperature muffle furnace serves as the critical reaction vessel for transforming raw chemical precursors into functional sensing materials. Specifically, it acts as the primary tool for annealing and calcination, thermally converting metal hydroxides or salts into stable metal oxides with the precise crystalline structures required for detecting glucose.
By applying precise thermal treatment in a controlled, contamination-free environment, the muffle furnace drives essential phase transitions—such as converting amorphous structures into active crystalline phases—which directly dictates the electrochemical catalytic activity of the final sensor.

The Mechanism of Material Transformation
To create an effective non-enzymatic sensor, you cannot simply use raw chemical precursors; they must be thermally processed to achieve the correct chemical identity.
Converting Precursors to Oxides
The primary function of the furnace is calcination.
It subjects precursors, such as metal hydroxides or metal salts, to high heat. This process drives off volatile components and chemically converts the precursor into a stable metal oxide.
Inducing Phase Transformations
Beyond simple conversion, the furnace dictates the crystallographic arrangement of the atoms.
Raw materials often start in an amorphous (disordered) state. The muffle furnace provides the energy required to rearrange these atoms into specific crystal phases.
Example: Titanium Dioxide Optimization
A prime example from the literature involves Titanium Dioxide (TiO2) nanotubes.
Initially, these may exist in an amorphous state. Through controlled heating, the furnace induces a phase transformation to the anatase phase, which is photochemically active and superior for sensing applications.
Enhancing Sensor Performance
The physical changes induced by the furnace translate directly to how well the sensor performs in a laboratory or clinical setting.
Maximizing Catalytic Activity
Non-enzymatic sensors rely on the material's surface to catalyze the oxidation of glucose.
Specific crystal phases, such as the anatase phase mentioned above, possess higher energy surfaces or more active sites. By locking in these phases, the furnace significantly enhances the electrochemical catalytic performance.
Ensuring Chemical Purity
Electrochemistry is highly sensitive to impurities.
The muffle furnace isolates the material from fuel combustion byproducts. This creates a contamination-free environment, ensuring that the sensor's signal comes from glucose interaction, not interference from impurities introduced during synthesis.
Understanding the Trade-offs
While high-temperature treatment is necessary, it introduces specific risks that must be managed to avoid degrading the sensor material.
The Risk of Over-Sintering
While heat improves crystallinity, excessive heat or duration can lead to sintering (densification).
For sensors, you want high surface area. If the material sinters too much, the particles fuse together, reducing the active surface area available for glucose detection.
Phase Instability
Temperature control must be precise.
Heating beyond the optimal range may push the material past the desired active phase into a more thermodynamically stable—but less catalytically active—phase (e.g., converting anatase entirely to rutile).
Making the Right Choice for Your Goal
The muffle furnace is not a "set it and forget it" tool; it is a variable that tunes your material's properties.
- If your primary focus is maximizing sensitivity: Prioritize temperatures that achieve the specific crystal phase (e.g., anatase) known for high catalytic activity, rather than simply maximizing crystallinity.
- If your primary focus is reproducibility: Ensure your furnace offers precise temperature ramp rates and hold times to guarantee that every batch undergoes the exact same phase transformation.
- If your primary focus is signal purity: Utilize the furnace's isolation capabilities to prevent combustion byproducts from contaminating the porous structure of your oxide.
Ultimately, the muffle furnace is the bridge between a raw chemical potential and a high-performance, electrochemically active device.
Summary Table:
| Process Step | Primary Function | Impact on Sensor Performance |
|---|---|---|
| Calcination | Converts precursors (hydroxides/salts) to oxides | Establishes the chemical identity and stability of the sensing material. |
| Annealing | Induces phase transformation (e.g., Amorphous to Anatase) | Maximizes electrochemical catalytic activity by optimizing crystal structure. |
| Thermal Isolation | Provides a contamination-free environment | Ensures high signal purity and prevents interference from impurities. |
| Controlled Heating | Manages sintering and particle fusion | Balances crystallinity with high surface area for better glucose detection. |
Elevate Your Material Research with KINTEK Precision
Achieving the perfect crystalline phase for non-enzymatic glucose sensors requires more than just heat—it requires absolute thermal control. KINTEK provides state-of-the-art Muffle, Tube, and Vacuum furnace systems designed to give researchers the precision needed for sensitive calcination and annealing processes.
Why choose KINTEK for your lab?
- Expert R&D & Manufacturing: Our systems are built for high-performance material transformation.
- Fully Customizable: Tailor temperature ramp rates and environments (CVD, Rotary, or Vacuum) to your unique synthesis needs.
- Superior Results: Ensure batch-to-batch reproducibility and eliminate contamination in your metal-oxide sensors.
Ready to optimize your sensing materials? Contact KINTEK today to discuss your project requirements!
Visual Guide
References
- Haibing Zhu, Zhanjun Yang. Non-Enzymatic Electrochemical Glucose Sensors Based on Metal Oxides and Sulfides: Recent Progress and Perspectives. DOI: 10.3390/chemosensors13010019
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- How is a laboratory muffle furnace used in 3D-printed PP-CF cross-linking? Achieve Thermal Stability at 150 °C
- How does automatic temperature control work in a muffle furnace? Ensure Precision and Stability for Your Lab
- What role does a high-temperature muffle furnace play in the green synthesis of TiO2? Key Phases for Pure Nanoparticles
- What safety features should be considered when selecting a muffle furnace? Ensure Lab Safety with Advanced Protection Systems
- How does a muffle furnace facilitate the final conversion of ZnO nanopowders? Precision Calcination for Pure Results
- What is the function of a high-temperature muffle furnace in HZSM-5 preparation? Master Catalytic Activation
- How do electrical muffle furnaces work? Unlock Precision Heating for Your Lab
- What is the maximum temperature of the muffle furnace? It's a critical design choice.



















