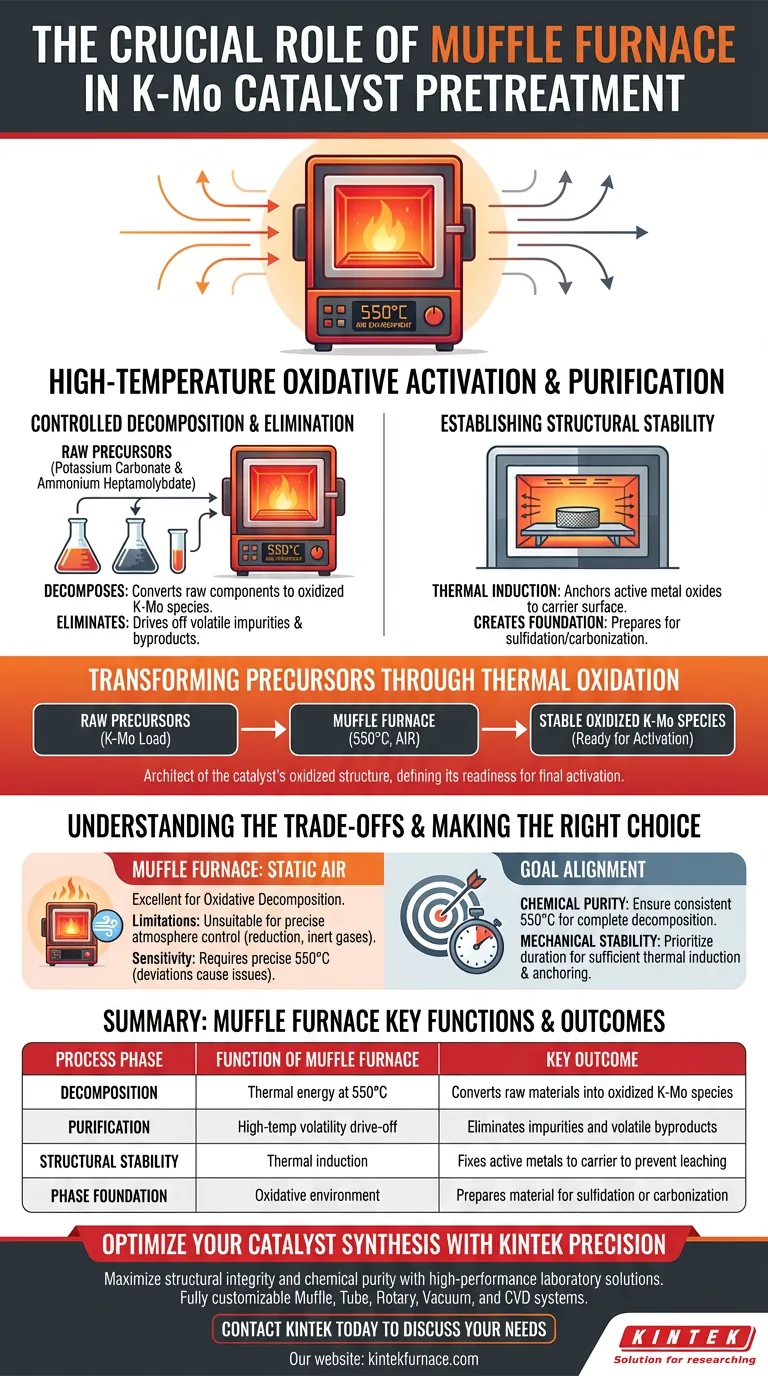
The Muffle Furnace serves as the primary vessel for high-temperature oxidative activation. specifically for K-Mo (Potassium-Molybdenum) catalyst precursors, it provides a stable 550°C air environment to decompose potassium carbonate and ammonium heptamolybdate. This thermal treatment is the pivotal step that converts raw, loaded components into stable, oxidized K-Mo species ready for further activation.
The Muffle Furnace functions as a controlled oxidation chamber that purifies precursors and fixes active metals to the carrier. By facilitating thermal decomposition at 550°C, it establishes the structural and chemical stability required for subsequent sulfidation or carbonization.
Transforming Precursors through Thermal Oxidation
Controlled Decomposition of Raw Materials
The primary function of the muffle furnace in this context is to induce the decomposition of specific raw materials: potassium carbonate and ammonium heptamolybdate.
Operating at 550°C, the furnace provides the thermal energy necessary to break down these compounds. This process effectively converts the precursor load into oxidized K-Mo oxides.
Elimination of Volatile Impurities
Beyond simple conversion, this high-temperature treatment acts as a purification step.
The heat drives off volatile byproducts and impurities inherent in the raw precursor mix. This ensures that the remaining material consists only of the desired metal oxides and the support carrier.
Establishing Structural Stability
Inducing Metal-Carrier Interactions
The heat provided by the muffle furnace does more than decompose chemicals; it alters the physical structure of the catalyst.
Through a process of thermal induction, the high temperature promotes a strong interaction between the active metal oxides and the surface of the carrier. This "anchoring" effect is vital for preventing metal leaching or sintering during later stages.
Creating a Stable Phase Basis
The ultimate goal of this pretreatment is to create a robust foundation for future processing.
By converting the precursors into stable oxides, the muffle furnace prepares the material for subsequent, more aggressive treatments, such as sulfidation or carbonization. Without this stabilization, the catalyst would lack the necessary phase integrity to perform effectively in reaction environments.
Understanding the Trade-offs
Atmosphere Limitations
It is critical to recognize that a standard muffle furnace typically operates with a static air atmosphere.
While excellent for oxidative decomposition (turning precursors into oxides), it is generally unsuitable for processes requiring precise atmosphere control, such as reduction or the introduction of inert gases. For those specific synthesis routes, a tube furnace would be the requisite equipment.
Sensitivity to Temperature Precision
The specific target of 550°C is not arbitrary; it is the thermal threshold required for K-Mo precursors.
Deviating significantly from this temperature can lead to issues. Temperatures that are too low may result in incomplete decomposition of the carbonates, while excessive heat could damage the carrier structure or cause unwanted sintering of the metal oxides.
Making the Right Choice for Your Goal
To maximize the effectiveness of your K-Mo catalyst preparation, align your furnace operations with your specific objectives:
- If your primary focus is Chemical Purity: Ensure the furnace maintains a consistent 550°C to guarantee the complete decomposition of ammonium and carbonate residues.
- If your primary focus is Mechanical Stability: Prioritize the duration of the heat treatment to allow sufficient time for thermal induction to fix the metal oxides to the carrier surface.
The Muffle Furnace is not just a heater; it is the architect of the catalyst's oxidized structure, defining its readiness for final activation.
Summary Table:
| Process Phase | Function of Muffle Furnace | Key Outcome |
|---|---|---|
| Decomposition | Thermal energy at 550°C | Converts raw materials into oxidized K-Mo species |
| Purification | High-temp volatility drive-off | Eliminates impurities and volatile byproducts |
| Structural Stability | Thermal induction | Fixes active metals to carrier to prevent leaching |
| Phase Foundation | Oxidative environment | Prepares material for sulfidation or carbonization |
Optimize Your Catalyst Synthesis with KINTEK Precision
Maximize the structural integrity and chemical purity of your K-Mo catalysts with KINTEK’s high-performance laboratory solutions. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your specific thermal threshold and atmosphere requirements.
Whether you need stable oxidative environments or precise atmosphere control for reduction, our furnaces ensure consistent thermal induction for your advanced material research. Contact KINTEK today to discuss your unique lab furnace needs and elevate your catalyst preparation efficiency.
Visual Guide
References
- Hao Wang, Yongming Luo. The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis. DOI: 10.3390/catal14030190
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What types of heating elements are used in muffle furnaces and their temperature ranges? Choose the Right Element for Your Lab
- What is the primary purpose of using a high-temperature box resistance furnace during the pretreatment of kaolin catalysts? Optimize Performance & Structure
- Why is isolation from contaminants important in a muffle furnace? Ensure Purity and Integrity in High-Temp Processes
- What are the advantages of box furnaces in terms of versatility? Unlock Flexibility for Diverse Material Processing
- What is a muffle furnace and how does it work? Discover Its Key Benefits for Your Lab
- What environmental testing applications involve muffle furnaces? Achieve Accurate Soil and Water Analysis
- What are the temperature capabilities of muffle furnaces? Find Your Perfect High-Temp Solution
- How are box type resistance furnaces used in metallic material R&D? Unlock Precise Heat Treatment and Alloy Development



















