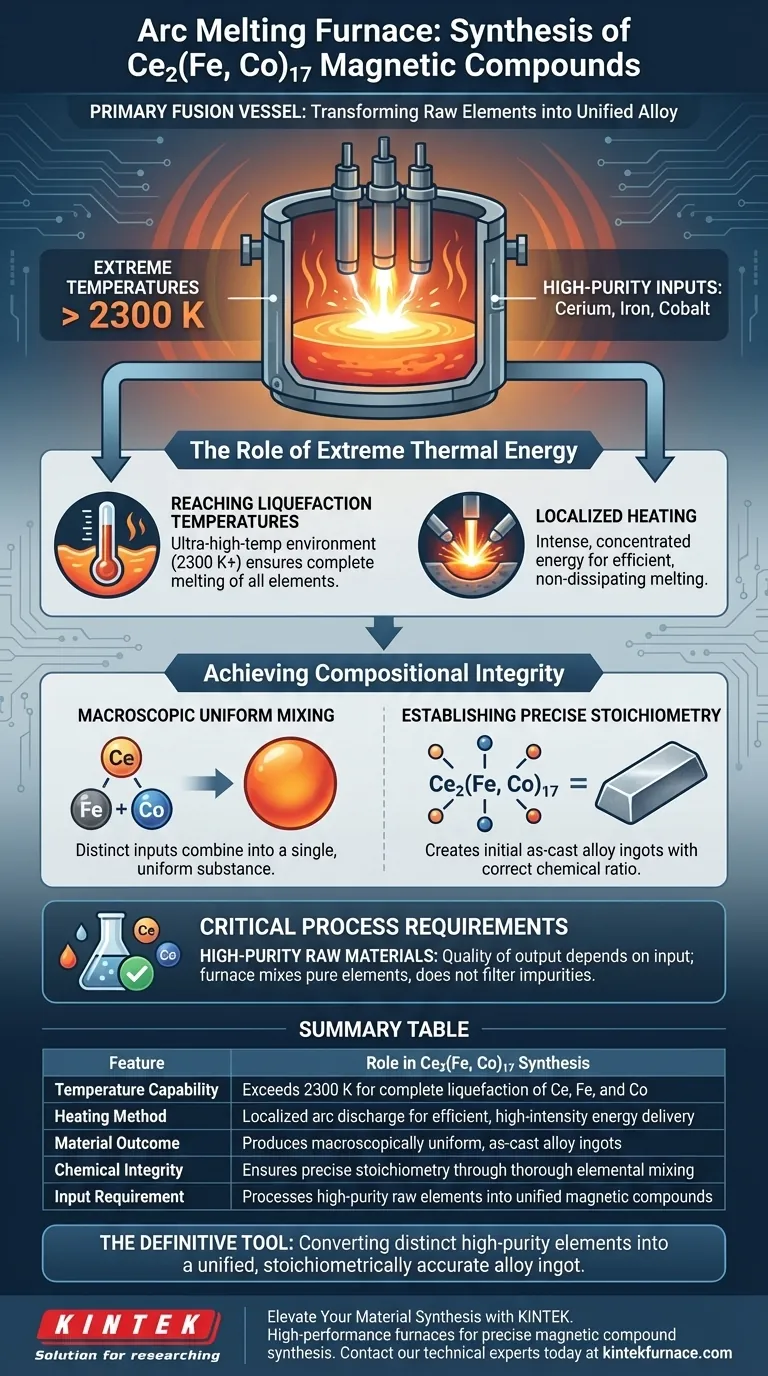
The arc melting furnace serves as the primary fusion vessel in the synthesis of Ce2(Fe, Co)17, acting as the critical first step to transform raw elements into a unified alloy. It creates a specific, localized environment capable of generating temperatures exceeding 2300 K, which is necessary to fully liquefy high-purity inputs like Cerium, Iron, and Cobalt.
The core function of the arc melting furnace is to overcome the high melting points of constituent elements to create a macroscopically uniform, as-cast ingot with precise chemical balance.

The Role of Extreme Thermal Energy
Reaching Liquefaction Temperatures
The synthesis of magnetic compounds requires energy levels that standard furnaces cannot easily achieve.
The arc melting furnace provides an ultra-high-temperature environment, typically exceeding 2300 K.
This extreme heat is non-negotiable for ensuring the complete melting of the raw elemental materials involved in the process.
Localized Heating
The heat generation in an arc melting furnace is described as localized.
This intense concentration of energy ensures that the target materials melt thoroughly without dissipating energy unnecessarily to the surrounding infrastructure.
Achieving Compositional Integrity
Macroscopic Uniform Mixing
Merely melting the elements is insufficient; they must be combined into a homogeneous mixture.
The furnace facilitates the thorough mixing of Cerium (Ce), Iron (Fe), and Cobalt (Co).
This ensures that the distinct elemental inputs lose their individual boundaries and become a single, uniform substance at the macroscopic level.
Establishing Precise Stoichiometry
The ultimate goal of this heating process is to lock in the correct chemical ratio of the compound.
By ensuring complete melting and mixing, the furnace produces initial as-cast alloy ingots that adhere to strict stoichiometric requirements.
This creates a reliable foundation for any subsequent processing steps required to finalize the magnetic material.
Critical Process Requirements
The Necessity of High-Purity Inputs
While the furnace provides the mechanism for mixing, the quality of the output depends heavily on the input.
The process explicitly requires high-purity raw elemental materials.
The furnace is designed to combine these pure elements; it does not filter impurities, so the starting quality dictates the integrity of the final Ce2(Fe, Co)17 compound.
Making the Right Choice for Your Goal
To leverage an arc melting furnace effectively for Ce2(Fe, Co)17 synthesis, consider your specific objectives:
- If your primary focus is material homogeneity: Ensure the furnace is operated at temperatures exceeding 2300 K to guarantee macroscopic uniform mixing of the Cobalt, Iron, and Cerium.
- If your primary focus is chemical accuracy: rely on the furnace's ability to fully melt the charge to produce as-cast ingots with precise stoichiometry.
The arc melting furnace is the definitive tool for converting distinct high-purity elements into a unified, stoichiometrically accurate alloy ingot.
Summary Table:
| Feature | Role in Ce2(Fe, Co)17 Synthesis |
|---|---|
| Temperature Capability | Exceeds 2300 K for complete liquefaction of Ce, Fe, and Co |
| Heating Method | Localized arc discharge for efficient, high-intensity energy delivery |
| Material Outcome | Produces macroscopically uniform, as-cast alloy ingots |
| Chemical Integrity | Ensures precise stoichiometry through thorough elemental mixing |
| Input Requirement | Processes high-purity raw elements into unified magnetic compounds |
Elevate Your Material Synthesis with KINTEK
Precise magnetic compound synthesis requires equipment that can withstand extreme thermal demands while maintaining chemical integrity. Backed by expert R&D and manufacturing, KINTEK offers high-performance Arc Melting, Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your unique laboratory needs.
Whether you are synthesizing Ce2(Fe, Co)17 or developing next-generation alloys, our specialized high-temp furnaces provide the uniformity and control your research deserves.
Ready to optimize your alloying process? Contact our technical experts today to find the perfect thermal solution for your lab.
Visual Guide
References
- H. Jaballah, Lotfi Bessais. Structural, Magnetic, and Magnetocaloric Properties of Ce2(Fe, Co)17 Compounds: Tuning Magnetic Transitions and Enhancing Refrigeration Efficiency. DOI: 10.3390/ma18091958
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
People Also Ask
- What is the role of shell mold heating in a vacuum induction furnace? Optimize Casting Flow & Integrity
- What technological advantages do modern induction melting solutions offer? Unlock Superior Metallurgical Quality & Efficiency
- What is the role of a vacuum induction furnace in CuNiSiCrCoTiNbx synthesis? Ensure Purity & Homogeneity
- What is the role of a laboratory-scale VIM furnace for carbide-free bainitic steel? High-Purity Ingot Development
- Importance of Induction Heating System and Coil Design in ODS Steel Bonding: Optimize Your Thermal Profile
- What is vacuum induction melting technology and why is it important? Achieve High-Purity Metals for Critical Applications
- What is the function of a Vacuum Induction Heating Furnace in research? Synthesis and Purity of Copper-Bearing Steel
- What are the environmental benefits of using an IGBT induction melting furnace? Boost Efficiency & Cut Emissions



















