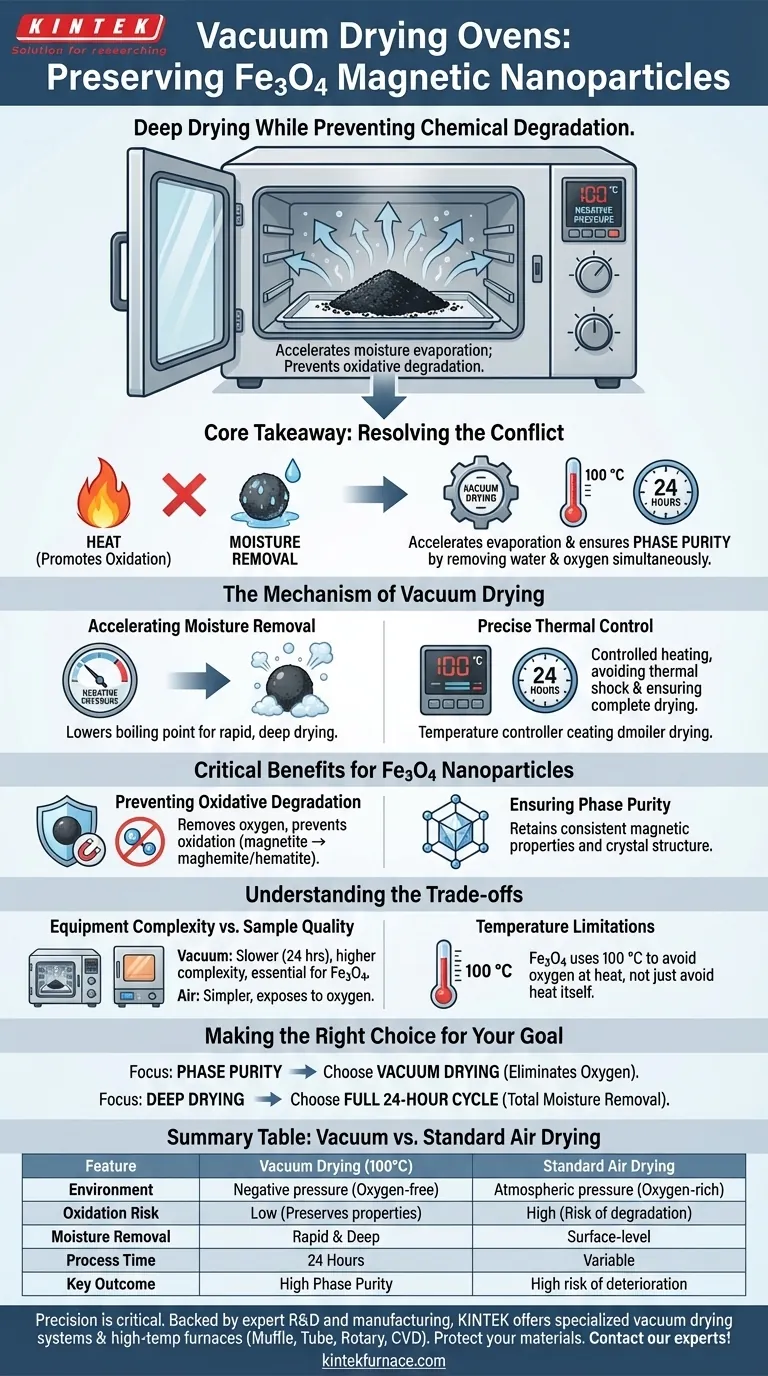
The primary role of a vacuum drying oven in processing Fe3O4 nanoparticles is to facilitate deep drying while preventing chemical degradation.
Specifically, the oven maintains a constant temperature of 100 °C under negative pressure for a duration of 24 hours. This environment accelerates the evaporation of moisture from wet precipitates without exposing the nanoparticles to the oxygen-rich environment that typically causes oxidative degradation at high temperatures.
Core Takeaway Drying magnetic nanoparticles presents a conflict: you need heat to remove moisture, but heat promotes oxidation which destroys magnetic properties. Vacuum drying resolves this by using negative pressure to accelerate evaporation, ensuring phase purity by removing water and oxygen simultaneously.

The Mechanism of Vacuum Drying
Accelerating Moisture Removal
The vacuum drying oven creates a negative pressure environment within the chamber. This lowers the boiling point of water and residual solvents trapped within the Fe3O4 precipitates.
By reducing the atmospheric pressure, the oven allows moisture to evaporate rapidly and thoroughly. This ensures deep drying of the material, which is difficult to achieve with standard air drying methods.
Precise Thermal Control
For Fe3O4 processing, the oven is typically set to a constant 100 °C. This temperature is sufficient to drive off water when combined with a vacuum, yet controlled enough to avoid thermal shock.
The process requires a sustained cycle, often lasting 24 hours, to ensure the precipitates are completely dry throughout their volume, not just on the surface.
Critical Benefits for Fe3O4 Nanoparticles
Preventing Oxidative Degradation
The most significant risk during the drying of Fe3O4 (magnetite) is oxidation. If exposed to high temperatures in the presence of air, Fe3O4 can oxidize into non-magnetic phases (such as maghemite or hematite).
The vacuum environment removes air from the chamber, effectively eliminating the oxygen source. This prevents oxidative degradation, preserving the chemical identity of the magnetite.
Ensuring Phase Purity
Because the vacuum prevents chemical changes during the drying process, the final powder retains high phase purity.
This means the magnetic properties and crystal structure of the resulting nanoparticles remain consistent with the synthesized material, rather than being altered by the post-processing steps.
Understanding the Trade-offs
Equipment Complexity vs. Sample Quality
Standard blast drying ovens use hot air circulation, which is simpler but exposes materials to oxygen. While effective for robust materials, this method risks chemical deterioration or agglomeration in sensitive nanomaterials.
Vacuum drying is a slower, more intensive process (24 hours) compared to rapid air drying. However, for materials like Fe3O4 where magnetic performance is dictated by chemical structure, the extra time and equipment complexity are necessary costs to avoid spoilage.
Temperature Limitations
While vacuum allows for lower-temperature drying generally, Fe3O4 still utilizes 100 °C.
In other contexts (like t-BTO or MXene), vacuum ovens are used to drop temperatures significantly (e.g., to 60–80 °C) to prevent thermal damage to organic groups. For Fe3O4, the vacuum is less about avoiding heat itself and more about avoiding oxygen at heat.
Making the Right Choice for Your Goal
When establishing a post-processing protocol for magnetic nanoparticles, align your method with your material's sensitivity:
- If your primary focus is Phase Purity: Prioritize vacuum drying to eliminate oxygen exposure and prevent the conversion of Fe3O4 to non-magnetic iron oxides.
- If your primary focus is Deep Drying: Commit to the full 24-hour cycle under negative pressure to ensure total moisture removal from the precipitate core.
The vacuum drying oven is not just a drying tool; it is a protective environment that locks in the chemical and magnetic potential of your nanoparticles.
Summary Table:
| Feature | Vacuum Drying (100°C) | Standard Air Drying |
|---|---|---|
| Environment | Negative pressure (Oxygen-free) | Atmospheric pressure (Oxygen-rich) |
| Oxidation Risk | Low (Preserves magnetic properties) | High (Risk of degradation to hematite) |
| Moisture Removal | Rapid & Deep evaporation | Surface-level or slow core drying |
| Process Time | 24 Hours (Consistent) | Variable (Potential for uneven drying) |
| Key Outcome | High Phase Purity | High risk of chemical deterioration |
Precision is critical when preserving the magnetic integrity of Fe3O4 nanoparticles. Backed by expert R&D and manufacturing, KINTEK offers specialized vacuum drying systems and a wide range of lab high-temp furnaces—including Muffle, Tube, Rotary, and CVD systems—all customizable for your unique research needs. Protect your materials from oxidative degradation and achieve superior phase purity today. Contact our experts to find your perfect furnace solution!
Visual Guide
References
- Yingtao Sun, Jianfeng Zhou. Developing and characterizing magnetic nanocomposites for effective metal ion removal in wastewater treatment. DOI: 10.46690/capi.2025.08.03
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- How does Thermogravimetric Analysis (TGA/DTG) provide industrial guidance? Optimize Blast Furnace Dust Treatment
- How does Faraday's Law of Induction work in induction heating? Achieve Precise, Non-Contact Thermal Processing
- What role does a laboratory blast drying oven play in metal powder preparation? Ensure Purity & Prevent Oxidation
- What is the importance of a stable thermal environment during crystallization? Ensure Precision in Metal Oxide Films
- What processes can continuous furnaces perform in a single step? Master Debinding and Sintering for High-Volume Production
- What is the function of an industrial drying oven in ZnZrOx catalyst prep? Ensure Uniform Metal Precursor Adsorption
- What is the purpose of annealing the sapphire substrate at 980 °C with Cr? Achieve Unidirectional Cr2S3 Growth
- How do industrial heat treatment furnaces ensure 55Si2 spring steel stability? Optimize Your Tempering Process



















