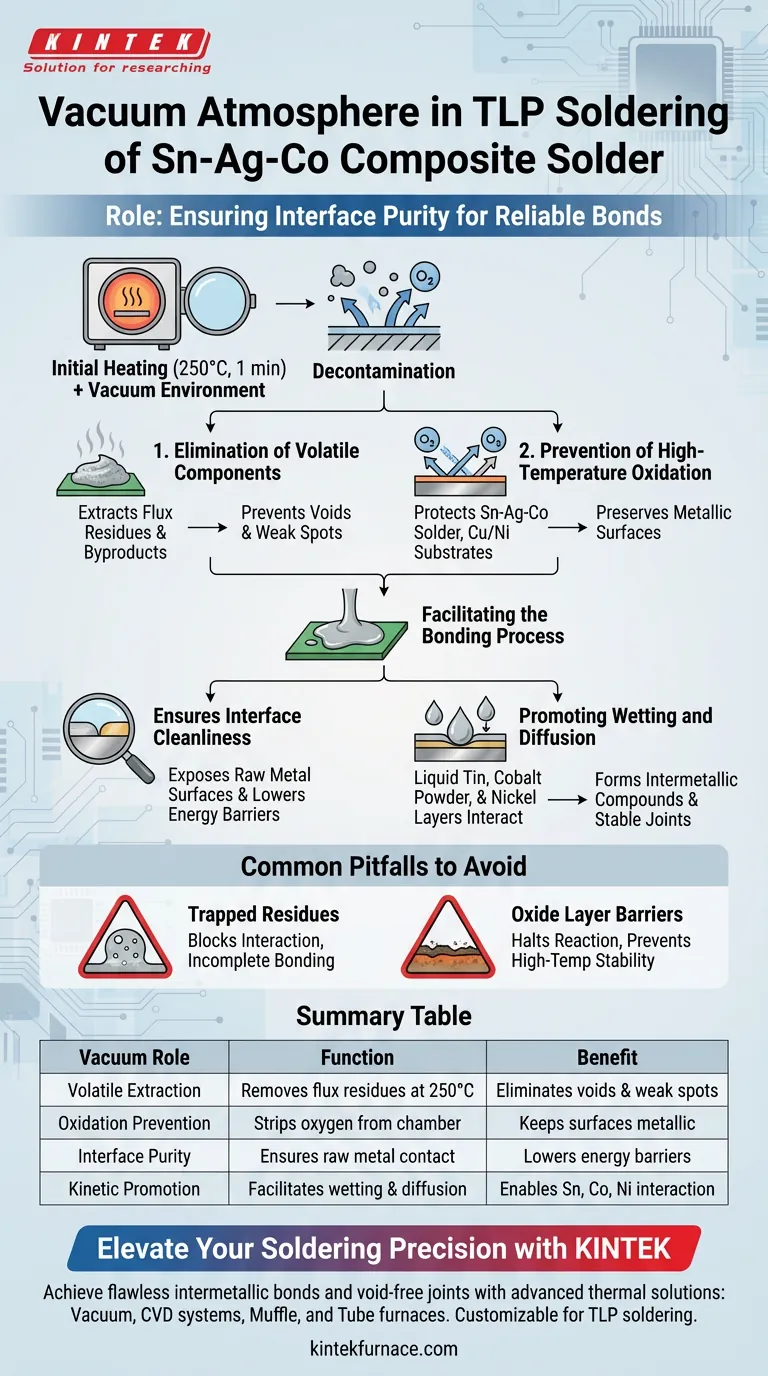
The role of a vacuum atmosphere is to ensure interface purity during the critical initial heating stage of Transient Liquid Phase (TLP) soldering. Specifically, heating the Sn-Ag-Co composite solder at 250 °C for one minute under vacuum eliminates volatile flux residues and prevents oxidation. This creates a pristine environment that allows liquid tin, cobalt powder, and nickel layers to interact chemically, securing a reliable bond.
The vacuum environment acts as a purification step, stripping away contaminants and oxygen to enable the essential wetting and diffusion reactions required for high-quality TLP joints.
The Mechanics of Decontamination
Elimination of Volatile Components
During the soldering process, the solder paste undergoes a significant physical transformation. The initial heating phase is designed to release volatile components trapped within the paste.
A vacuum atmosphere actively extracts these volatiles, most notably flux residues. Removing these byproducts early prevents them from becoming trapped in the final joint, which could otherwise lead to voids or weak spots.
Prevention of High-Temperature Oxidation
Heat naturally accelerates oxidation, which is detrimental to soldering. The vacuum environment removes oxygen from the process chamber.
This prevents high-temperature oxidation of two critical elements: the Sn-Ag-Co solder material itself and the copper or nickel interfaces on the substrate. Preserving these metal surfaces in their metallic state is essential for the chemical reactions that follow.
Facilitating the Bonding Process
Ensuring Interface Cleanliness
For TLP soldering to work, the liquid phase must react with the solid phase. A vacuum ensures the cleanliness of the interface between the solder and the substrate.
By removing physical contaminants (volatiles) and chemical barriers (oxides), the vacuum exposes the raw metal surfaces. This lowers the surface energy barriers that typically inhibit bonding.
Promoting Wetting and Diffusion
A clean, oxide-free surface allows for superior wetting. The liquid solder can spread uniformly across the substrate without beading up.
More importantly, this contact promotes diffusion reactions. In this specific alloy system, the vacuum enables the necessary chemical interaction between the liquid tin, the cobalt powder suspended in the solder, and the nickel layers of the substrate.
Common Pitfalls to Avoid
The Risk of Trapped Residues
If the vacuum is insufficient or the heating duration is too short, flux residues may not fully evaporate.
These trapped residues act as contaminants. They physically block the interaction between the tin and the cobalt/nickel, leading to incomplete bonding and reduced mechanical strength.
The Barrier of Oxide Layers
Attempting this process in an air or inert atmosphere with high oxygen content can be fatal to the joint.
Even thin oxide layers on the nickel or copper substrates act as diffusion barriers. These barriers halt the reaction between the liquid tin and the substrate, preventing the formation of the intermetallic compounds that give TLP joints their high-temperature stability.
Making the Right Choice for Your Process
To maximize the reliability of Sn-Ag-Co TLP joints, consider these specific goals:
- If your primary focus is Void Reduction: Ensure the initial heating stage at 250 °C is held for at least one minute under vacuum to allow complete outgassing of flux volatiles.
- If your primary focus is Intermetallic Formation: Prioritize a high-quality vacuum to prevent oxidation on the nickel layers, ensuring nothing impedes the diffusion of liquid tin and cobalt.
The vacuum is not just a passive environment; it is an active tool that prepares the metallurgy for a successful bond.
Summary Table:
| Vacuum Role | Function | Benefit |
|---|---|---|
| Volatile Extraction | Removes flux residues at 250 °C | Eliminates voids and weak spots |
| Oxidation Prevention | Strips oxygen from the chamber | Keeps solder and substrate surfaces metallic |
| Interface Purity | Ensures raw metal-to-metal contact | Lowers surface energy barriers for bonding |
| Kinetic Promotion | Facilitates wetting and diffusion | Enables chemical interaction of Sn, Co, and Ni |
Elevate Your Soldering Precision with KINTEK
Achieve flawless intermetallic bonds and void-free joints with our advanced thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers state-of-the-art Vacuum, CVD systems, Muffle, and Tube furnaces—all customizable to meet the rigorous demands of your TLP soldering and high-temp lab processes. Don't let oxidation compromise your research. Contact us today to find the perfect system for your unique needs!
Visual Guide
References
- Byungwoo Kim, Yoonchul Sohn. Transient Liquid Phase Bonding with Sn-Ag-Co Composite Solder for High-Temperature Applications. DOI: 10.3390/electronics13112173
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- How does an industrial-grade high-temperature vertical furnace contribute to the homogenization annealing of magnetocaloric materials?
- Why is short-term annealing followed by water quenching necessary for Ti-15Mo alloys? Lock in Peak Material Performance
- What is the burnout cycle on a vacuum furnace? A Key to High-Purity Heat Treating
- What are the advantages of vacuum brazing aluminum compared to traditional welding methods? Superior Joint Integrity and Precision
- What are the key characteristics of high-temperature vacuum furnaces? Achieve Ultimate Purity and Precision in Material Processing
- What are the environmental requirements for vacuum sintering? Achieve Superior Material Density and Purity
- What are the technical advantages of using a vacuum drying oven for electrocatalyst powders? Pt/HCCP Drying Guide
- What role does a vacuum drying oven play in high-entropy alloy powder preparation? Ensure Peak Sintering Density



















