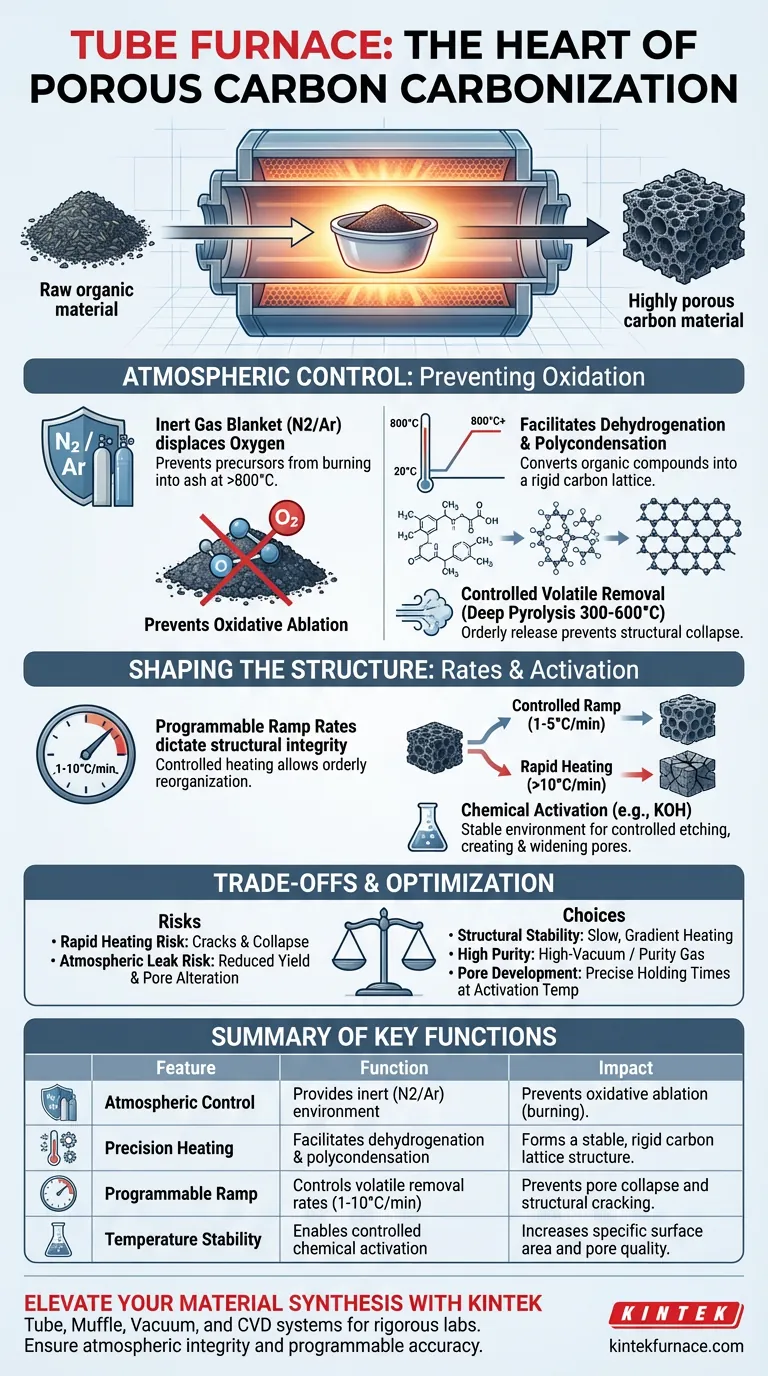
A tube furnace serves as the definitive control environment required to convert raw organic materials into high-quality porous carbon. It functions as a sealed reaction vessel that simultaneously provides a strictly inert atmosphere and executes precise thermal programs, ensuring the precursor material transforms chemically rather than simply burning away.
The tube furnace’s primary value lies in its ability to decouple heating from oxidation. By maintaining an oxygen-free zone while ramping temperatures to 800°C or higher, it forces materials to undergo dehydrogenation and polycondensation—stripping away volatiles to leave behind a stable, interconnected carbon skeleton.
The Critical Role of Atmospheric Control
Preventing Oxidative Ablation
The most immediate function of the tube furnace is to act as a barrier against oxygen. At the high temperatures required for carbonization (often 800°C or higher), carbon precursors are highly reactive.
Without a protective atmosphere, these materials would undergo oxidative ablation, essentially burning into ash and gas.
Creating an Inert Environment
The furnace utilizes a continuous flow of inert gases, primarily nitrogen or argon. This creates a "blanket" around the material.
By displacing oxygen, the furnace forces the material to decompose internally rather than react externally. This is the foundational step that allows for the retention of the carbon mass necessary for a porous structure.
Driving Chemical Transformation Through Precision Heating
Facilitating Dehydrogenation and Polycondensation
The tube furnace is not just a heater; it is a driver of specific chemical reactions. As the temperature rises, the furnace facilitates the dehydrogenation of precursors like petroleum pitch or biomass.
Simultaneously, it drives polycondensation, where small molecules join to form larger, more complex chains. This process rearranges the atomic structure, converting organic compounds into a rigid carbon lattice.
Controlling Volatile Removal (Deep Pyrolysis)
Between 300°C and 600°C, the furnace manages the removal of volatile components. This stage, known as deep pyrolysis, must be controlled carefully.
The furnace's precise heating rates ensure that volatiles are released in an orderly fashion. This controlled release prevents the destruction of the forming pore structure and results in a stable carbon framework.
Shaping the Final Carbon Structure
Precise Heating Rates
The structural integrity of porous carbon is dictated by how fast it is heated. The tube furnace enables programmable heating rates, typically ranging from 1°C to 10°C per minute (with 5°C/min being a common standard).
A controlled ramp rate is vital. It allows for the orderly reorganization of the carbon skeleton and prevents structural collapse that can occur if volatiles are expelled too violently.
Enabling Chemical Activation
When chemical activators (such as KOH or KCl) are used, the tube furnace provides the stable thermal environment needed for controlled etching.
By holding specific temperatures, the furnace allows these agents to react with the carbon framework. This creates and widens pores, directly influencing the material's specific surface area and final quality.
Understanding the Trade-offs
Sensitivity to Heating Rates
While the furnace allows for rapid heating, utilizing high ramp rates (e.g., above 10°C/min) can be detrimental. Rapid heating may cause the material to crack or the pores to collapse due to the explosive release of internal gases.
Atmospheric Integrity Risks
The quality of the final product is entirely dependent on the seal of the tube. Even a microscopic leak in the system can introduce oxygen.
Trace amounts of oxygen at 800°C will not just degrade the surface; they can alter the pore size distribution and significantly reduce the yield of the carbonization process.
Making the Right Choice for Your Goal
The configuration of your tube furnace process should depend on the specific properties you need in your porous carbon.
- If your primary focus is Structural Stability: Prioritize a slow, gradient heating rate (e.g., 1°C to 2°C/min) to allow for orderly atomic rearrangement and minimize thermal shock.
- If your primary focus is High Purity: Ensure the use of a high-vacuum tube furnace or high-purity argon gas to eliminate all traces of oxygen and prevent oxidative contamination.
- If your primary focus is Pore Development: Utilize a program with precise holding times at activation temperatures (e.g., 700°C) to allow chemical activators sufficient time to etch the carbon framework.
Success in carbonization is not just about reaching a high temperature; it is about the precision of the journey to get there.
Summary Table:
| Feature | Function in Carbonization | Impact on Final Product |
|---|---|---|
| Atmospheric Control | Provides inert (N2/Ar) environment | Prevents oxidative ablation (burning into ash) |
| Precision Heating | Facilitates dehydrogenation & polycondensation | Forms a stable, rigid carbon lattice structure |
| Programmable Ramp | Controls volatile removal rates (1-10°C/min) | Prevents pore collapse and structural cracking |
| Temperature Stability | Enables controlled chemical activation (e.g., KOH) | Increases specific surface area and pore quality |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between high-quality porous carbon and wasted precursors. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems specifically designed for rigorous lab environments. Backed by expert R&D and manufacturing, our high-temp furnaces offer the atmospheric integrity and programmable accuracy needed to ensure stable carbon skeletons and optimal pore development.
Whether you need a standard setup or a fully customizable solution for unique research needs, our team is ready to assist.
Contact KINTEK today to optimize your carbonization process!
Visual Guide
References
- Hui Liu, Qingshan Zhao. A Palladium Catalyst Supported on Boron-Doped Porous Carbon for Efficient Dehydrogenation of Formic Acid. DOI: 10.3390/nano14060549
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why are atmospheric controls important in horizontal tube furnaces? Ensure Precise Chemical Processing and Safety
- What role does a high-temperature tube furnace play in N-CP synthesis? Mastering Precision Carbonization
- What physical conditions do high-temperature tube furnaces provide for flue gas kinetics? Precision Thermal Simulation
- How does a tubular furnace contribute to the conversion of Co-Fe-ZIF precursors into Co-Fe-NC catalysts?
- What core functions does a high-temperature tube furnace provide? Master TiN-Carbon Composite Pyrolysis
- What temperature-related capabilities make multi zone tube furnaces valuable for research? Unlock Precision Thermal Control
- What is the critical role of a tube furnace in the preparation of beta-PbO powder? Optimize Lead-Acid Battery Recycling
- What role does an atmosphere-controlled vacuum tube furnace play in sintering? Mastering Porous Stainless Steel



















