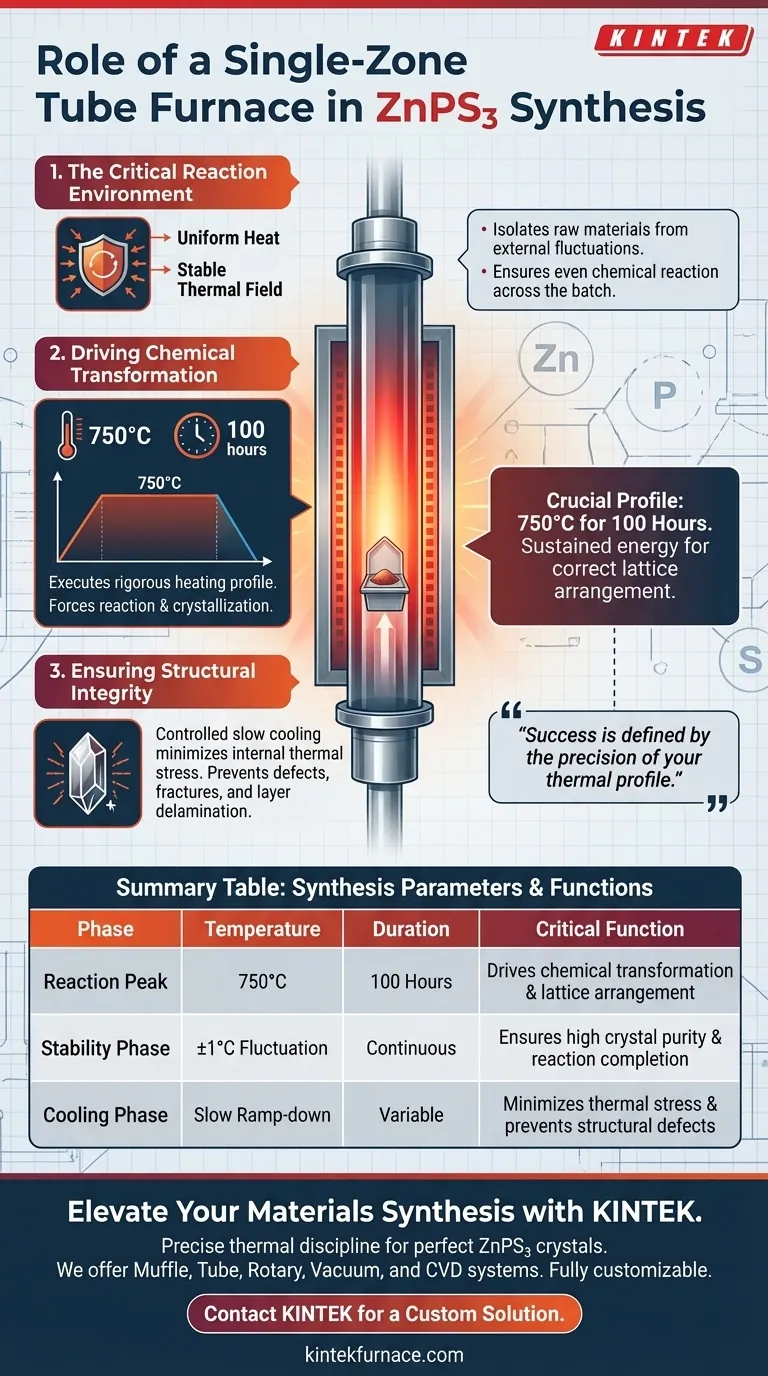
A single-zone tube furnace serves as the critical reaction environment for synthesizing ZnPS3, providing the precise thermal control necessary to transform raw materials into a layered crystal. It executes a rigorous heating profile—specifically maintaining high temperatures for extended periods—to drive the chemical reaction and facilitate the slow crystallization required for structural integrity.
The synthesis of ZnPS3 relies on stability rather than just intensity; the tube furnace ensures a consistent thermal field to drive the reaction and a controlled cooling phase to prevent defects, resulting in crystals with ideal physical dimensions.

The Mechanics of Thermal Synthesis
Creating the Reaction Environment
The primary function of the single-zone tube furnace is to generate a precisely controlled high-temperature thermal field.
Because ZnPS3 synthesis requires a stable environment, the furnace isolates the raw materials from external fluctuations. This uniform heat ensures that the chemical reaction proceeds evenly across the entire sample batch.
Driving Chemical Transformation
The furnace does not simply heat the material; it executes a specific heating program designed to force the reaction and subsequent crystallization.
For ZnPS3, this typically involves heating the chamber to 750°C and maintaining this temperature strictly for 100 hours. This sustained thermal energy provides the thermodynamic push required for the raw materials to combine and arrange into the correct crystal lattice.
Ensuring Structural Integrity
Once the reaction is complete, the furnace plays a vital role in the cooling process.
The equipment is programmed to cool the sample slowly rather than exposing it to rapid temperature drops. This controlled descent minimizes internal thermal stress within the newly formed material. By reducing stress, the furnace ensures the resulting ZnPS3 crystals possess a complete, unfragmented layered structure.
Operational Considerations and Constraints
The Necessity of Time
The process described is inherently time-intensive, requiring over four days of continuous operation at peak temperature.
Attempting to accelerate this process by shortening the 100-hour hold time can result in incomplete reactions or poor crystallization. The furnace must be capable of maintaining distinct stability over this long duration without temperature drift.
The Impact of Cooling Rates
The quality of the final physical dimensions is directly tied to the cooling phase.
If the furnace cools the sample too quickly, the material may suffer from thermal shock. This often leads to fractures or distortions in the layered structure, rendering the synthesis unsuccessful for high-precision applications.
Achieving Optimal Synthesis Results
To maximize the quality of your ZnPS3 yield, align your furnace programming with your specific outcome requirements:
- If your primary focus is Crystal Purity: Ensure the furnace can maintain the 750°C hold temperature with less than ±1°C fluctuation for the full 100-hour duration to ensure a complete reaction.
- If your primary focus is Structural Integrity: Prioritize the programming of the cooling ramp, ensuring it is slow enough to eliminate internal thermal stress and prevent layer delamination.
Success in synthesizing ZnPS3 is defined by the precision of your thermal profile; the furnace is merely the tool that enforces this discipline.
Summary Table:
| Synthesis Phase | Temperature Requirement | Duration | Critical Function |
|---|---|---|---|
| Reaction Peak | 750°C | 100 Hours | Drives chemical transformation & lattice arrangement |
| Stability Phase | ±1°C Fluctuation | Continuous | Ensures high crystal purity and reaction completion |
| Cooling Phase | Slow Ramp-down | Variable | Minimizes thermal stress and prevents structural defects |
Elevate Your Materials Synthesis with KINTEK
Precise thermal discipline is the difference between a fragmented sample and a perfect ZnPS3 crystal. KINTEK provides high-performance tube furnaces specifically engineered for long-duration stability and programmable cooling ramps.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory requirements. Whether you are synthesizing 2D materials or advanced ceramics, our equipment ensures the structural integrity your research demands.
Ready to optimize your high-temperature processes? Contact KINTEK today for a custom solution.
Visual Guide
References
- Abhishek Mukherjee, Svetlana V. Boriskina. Thermal and Dimensional Stability of Photocatalytic Material ZnPS<sub>3</sub> Under Extreme Environmental Conditions. DOI: 10.1002/aelm.202500093
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- What is the role of a Tube Furnace or Rotary Furnace in hydrogen reduction roasting? Optimize Lithium Recovery Efficiency.
- What core functions does a tube high-temperature furnace perform? Mastering In-situ Carbothermal Reduction
- What optional features are available for tube furnaces? Enhance Your Materials Processing with Precision Control
- How does a tube furnace facilitate the structural stabilization of lignin? Mastering Lignin-to-Carbon Transformation
- How does a laboratory tube furnace contribute to the continuity and quality of Mn3O4 arrays? Master Atomic Stitching
- What environmental conditions does a tube furnace provide for CFeS aerogels? Master Carbonization & Inert Protection
- What is the primary role of a Tube Furnace in g-C3N4 synthesis? Achieve Precise Thermal Polycondensation
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab



















