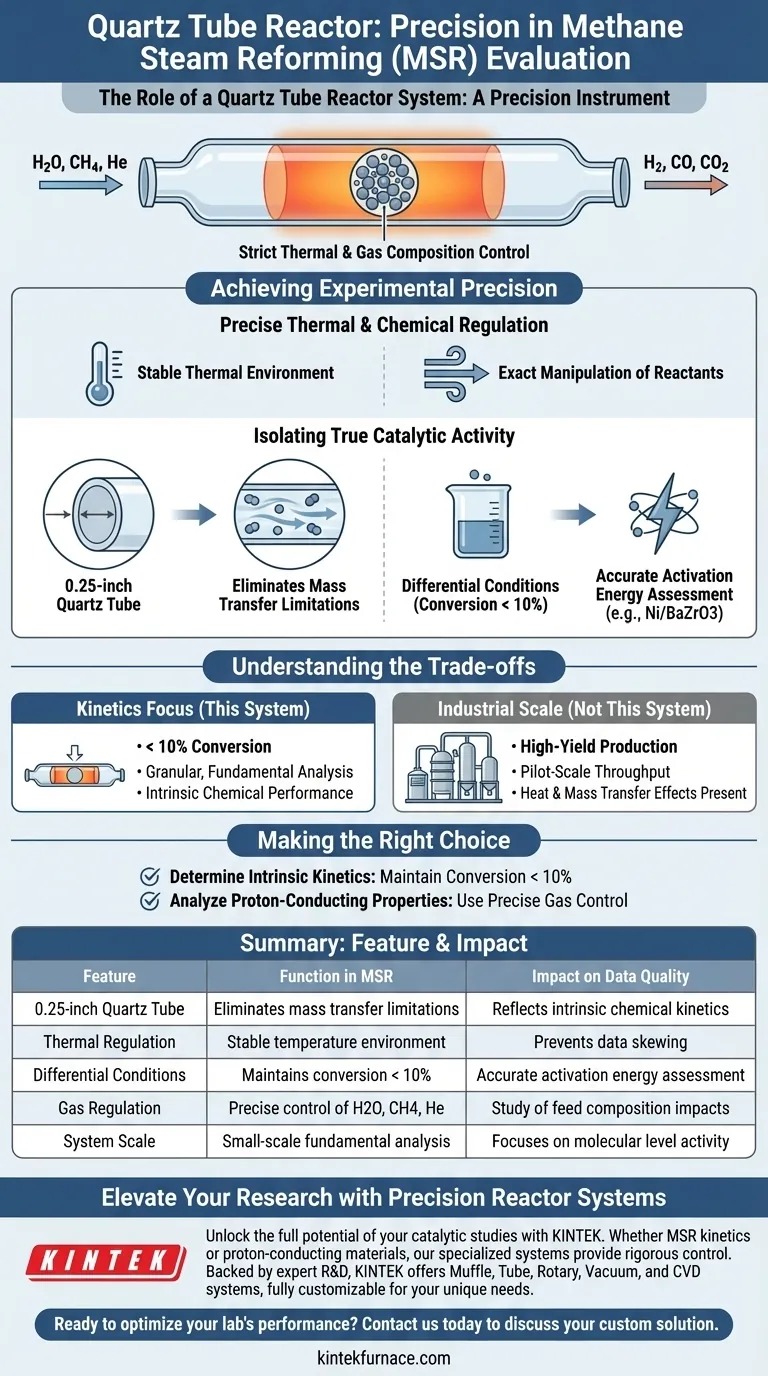
A quartz tube reactor system functions as a precision instrument for isolating the intrinsic chemical performance of catalysts during Methane Steam Reforming (MSR). It provides the strictly controlled thermal environment and gas composition regulation necessary to distinguish between true catalytic activity and physical transport effects.
By utilizing a 0.25-inch quartz tube to maintain differential reaction conditions, this system eliminates mass transfer limitations, ensuring that observed performance data reflects the catalyst's specific activation energy rather than external physical constraints.

Achieving Experimental Precision
To accurately evaluate MSR performance, you must first eliminate variables that distort data. The quartz tube reactor addresses this through rigorous environmental control.
Precise Thermal and Chemical Regulation
The primary function of the system is to provide a stable thermal environment. Fluctuations in temperature can skew kinetic data, making stability essential for reliable results.
Controlling Reactant Ratios
The system allows for the exact manipulation of reacting gases, specifically H2O, CH4, and He. This control is critical for studying how specific changes in feed composition impact the reforming process.
Isolating True Catalytic Activity
The "Deep Need" in MSR evaluation is to see how the catalyst performs at a molecular level, unclouded by the physics of how gases move through a reactor.
Eliminating Mass Transfer Limitations
A critical feature of this system is the use of a 0.25-inch quartz tube. This specific dimension helps create an environment where the resistance to gas diffusion (mass transfer limitations) is effectively removed.
Maintaining Differential Reaction Conditions
To ensure data accuracy, the reactor is operated under differential reaction conditions. This means keeping conversion rates below 10%.
Assessing Activation Energy
By removing physical limitations and keeping conversion low, researchers can accurately assess specific activation energy changes. This is particularly relevant for analyzing the proton-conducting properties of materials like Ni/BaZrO3 catalysts.
Understanding the Trade-offs
While this system is ideal for kinetic studies, it imposes specific operational constraints that differ from industrial applications.
Limited Conversion Rates
The requirement to maintain differential conditions means you are restricted to low conversion rates (<10%). You cannot use this specific setup to test high-yield production scenarios, as that would introduce the very heat and mass transfer effects you are trying to avoid.
Scale Constraints
The reliance on a 0.25-inch tube limits the volume of catalyst that can be tested. This is a tool for granular, fundamental analysis, not for pilot-scale throughput testing.
Making the Right Choice for Your Goal
When designing your MSR experiments, align your reactor settings with your specific analytical objectives.
- If your primary focus is determining intrinsic kinetics: Ensure you maintain conversion rates below 10% to eliminate mass transfer limitations.
- If your primary focus is analyzing proton-conducting properties: Use the precise gas control to isolate how the catalyst (e.g., Ni/BaZrO3) responds to specific activation energy changes.
Accurate MSR evaluation relies not just on the catalyst you choose, but on the rigorous isolation of variables that a quartz tube system provides.
Summary Table:
| Feature | Function in MSR Evaluation | Impact on Data Quality |
|---|---|---|
| 0.25-inch Quartz Tube | Eliminates mass transfer limitations | Ensures data reflects intrinsic chemical kinetics |
| Thermal Regulation | Provides stable temperature environment | Prevents data skewing from thermal fluctuations |
| Differential Conditions | Maintains conversion rates < 10% | Allows for accurate assessment of activation energy |
| Gas Regulation | Precise control of H2O, CH4, and He | Enables study of feed composition impacts |
| System Scale | Small-scale fundamental analysis | Focuses on molecular level activity vs. physical transport |
Elevate Your Research with Precision Reactor Systems
Unlock the full potential of your catalytic studies with KINTEK. Whether you are analyzing Methane Steam Reforming (MSR) kinetics or developing advanced proton-conducting materials, our specialized laboratory systems provide the rigorous control your data demands.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of high-performance equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems. All our laboratory high-temp furnaces are fully customizable to meet your unique experimental needs, ensuring you achieve the precise thermal and chemical environments necessary for groundbreaking results.
Ready to optimize your lab's performance? Contact us today to discuss your custom solution and experience the KINTEK advantage in precision engineering.
Visual Guide
References
- Kai Shen, John M. Vohs. Enhanced Methane Steam Reforming Over Ni/BaZrO3. DOI: 10.1007/s10562-025-05087-5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How is a laboratory tube furnace utilized to convert metal-organic precursors? Master Thin Film Pyrolysis Today
- How does a high-temperature tube furnace facilitate the conversion of Cu@ZIF-8? Master Precision Material Synthesis
- What option is available for frequently relocated split tube furnaces? Discover the Vertical Portable Stand Solution
- What is the role of mixing precursor with sulfur powder? Master Sulfidation in Tube Furnaces for Fe7S8@CT-NS
- What role does a single-zone tube furnace play in synthesizing ZnPS3? Master the Thermal Profile for Layered Materials
- Why is a tube furnace equipped with an atmosphere control system required for synthesizing h-Zn-Co-O solid solutions?
- What is the specific role of a Tube Furnace in phosphate/graphene annealing? Unlock High-Performance Electrode Synthesis
- What is the primary function of a tube furnace? Achieve Precise Atmospheric Control for Material Processing



















