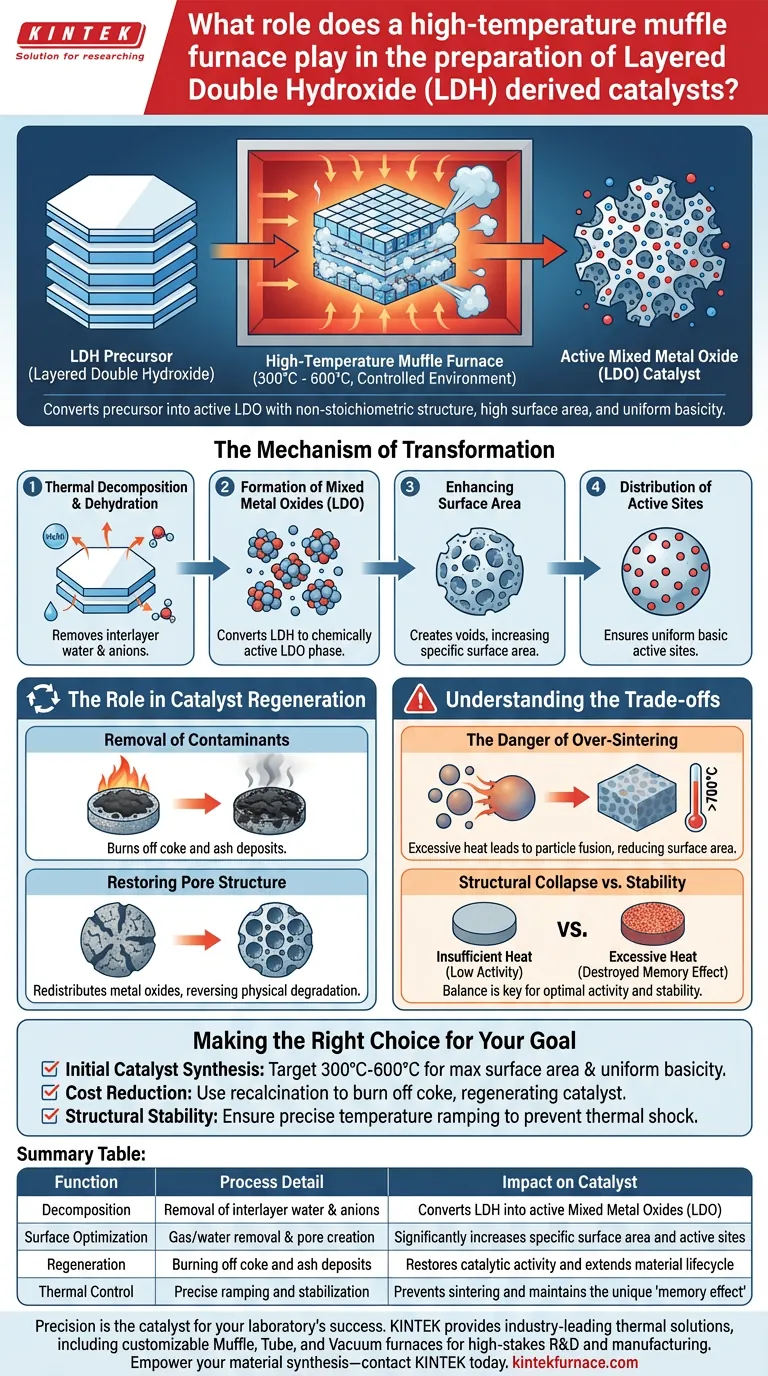
A high-temperature muffle furnace serves as the primary instrument for transforming Layered Double Hydroxide (LDH) precursors into active Mixed Metal Oxide (LDO) catalysts.
By providing a strictly controlled thermal environment, typically between 300°C and 600°C, the furnace drives the calcination process. This thermal treatment is essential for decomposing the precursor material, removing structural water and anions, and stabilizing the active sites required for catalytic reactions.
The muffle furnace does not simply dry the material; it fundamentally alters its chemical architecture. It converts the precursor into a non-stoichiometric structure with high specific surface area and uniformly distributed basicity, which are the defining characteristics of an effective LDH-derived catalyst.
The Mechanism of Transformation
Thermal Decomposition and Dehydration
The primary function of the furnace is to induce thermal decomposition. As the temperature rises, the furnace drives off interlayer water molecules (dehydration) and decomposes the anions residing between the structural layers.
Formation of Mixed Metal Oxides (LDO)
This decomposition converts the original LDH structure into a Mixed Metal Oxide (LDO). This phase transformation is critical because the resulting LDO possesses a non-stoichiometric structure, which is chemically more active than the stable precursor.
Enhancing Surface Area
The removal of gases and water during calcination creates voids within the material. This process significantly increases the specific surface area of the catalyst, providing more contact points for reactants during subsequent chemical processes.
Distribution of Active Sites
The furnace ensures the uniform distribution of active basic sites. A consistent thermal environment prevents "hot spots" during synthesis, ensuring that the catalytic activity is homogeneous across the entire material batch.
The Role in Catalyst Regeneration
Removal of Contaminants
Beyond initial synthesis, the muffle furnace is vital for recycling catalysts used in processes like biomass conversion. It provides the heat necessary to burn off coke deposits (carbon deposition) and ash that accumulate on the catalyst surface and block active sites.
Restoring Pore Structure
Recalcination in the furnace allows for the redistribution of metal oxides. This effectively restores the catalyst's pore structure, reversing the physical degradation that occurs during operation and extending the material's lifecycle.
Understanding the Trade-offs
The Danger of Over-Sintering
While high temperatures are necessary for activation, excessive heat can be detrimental. If the furnace temperature exceeds the optimal range (often above 600°C-700°C for certain LDH types), the material may undergo sintering.
Loss of Surface Area
Sintering causes the active particles to fuse together, causing a collapse of the porous structure. This drastically reduces the specific surface area and, consequently, the catalytic efficiency.
Structural Collapse vs. Stability
There is a delicate balance between stabilizing the structure and destroying it. Insufficient heat fails to remove all anions, leading to low activity, while excessive heat destroys the unique "memory effect" and basicity of the LDO structure.
Making the Right Choice for Your Goal
To maximize the utility of your muffle furnace in LDH catalyst preparation, consider your specific objective:
- If your primary focus is Initial Catalyst Synthesis: Target the 300°C to 600°C range to maximize surface area and create uniform basic sites without inducing sintering.
- If your primary focus is Cost Reduction: Utilize the furnace for recalcination to burn off coke deposits, regenerating the catalyst for multiple operational cycles.
- If your primary focus is Structural Stability: Ensure precise temperature ramping to prevent thermal shock, which can cause peeling or deactivation of the catalyst surface.
The muffle furnace is the tool that bridges the gap between a chemically inert precursor and a highly active, industrial-grade catalyst.
Summary Table:
| Function | Process Detail | Impact on Catalyst |
|---|---|---|
| Decomposition | Removal of interlayer water & anions | Converts LDH into active Mixed Metal Oxides (LDO) |
| Surface Optimization | Gas/water removal & pore creation | Significantly increases specific surface area and active sites |
| Regeneration | Burning off coke and ash deposits | Restores catalytic activity and extends material lifecycle |
| Thermal Control | Precise ramping and stabilization | Prevents sintering and maintains the unique 'memory effect' |
Precision is the catalyst for your laboratory's success. KINTEK provides industry-leading thermal solutions, including customizable Muffle, Tube, and Vacuum furnaces designed specifically for high-stakes R&D and manufacturing. Our systems ensure uniform heat distribution and precise temperature control, essential for achieving the perfect calcination of LDH precursors without the risk of sintering. Empower your material synthesis—contact KINTEK today for expert consultation.
Visual Guide
References
- Sivashunmugam Sankaranarayanan, Wangyun Won. Catalytic pyrolysis of biomass to produce bio‐oil using layered double hydroxides (<scp>LDH</scp>)‐derived materials. DOI: 10.1111/gcbb.13124
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is temperature control important in a muffle furnace? Ensure Accurate, Repeatable Results
- How does a muffle furnace work? A Guide to Clean, Uniform Heat Treatment
- How do high-vacuum muffle furnaces or tube furnaces facilitate the activation of materials like UiO-66-NH2?
- What is the function of a high-temperature muffle furnace in the columbite precursor method? Pure Perovskite Synthesis
- How are box type resistance furnaces used in metallic material R&D? Unlock Precise Heat Treatment and Alloy Development
- What is the temperature of a muffle oven? A Guide to Choosing the Right Range for Your Lab
- How do box type resistance furnaces contribute to catalytic material preparation? Unlock Precision in Catalyst Synthesis
- What core role does a high-temperature box resistance furnace play in the production of doped Nickel Oxide nanopowders?



















