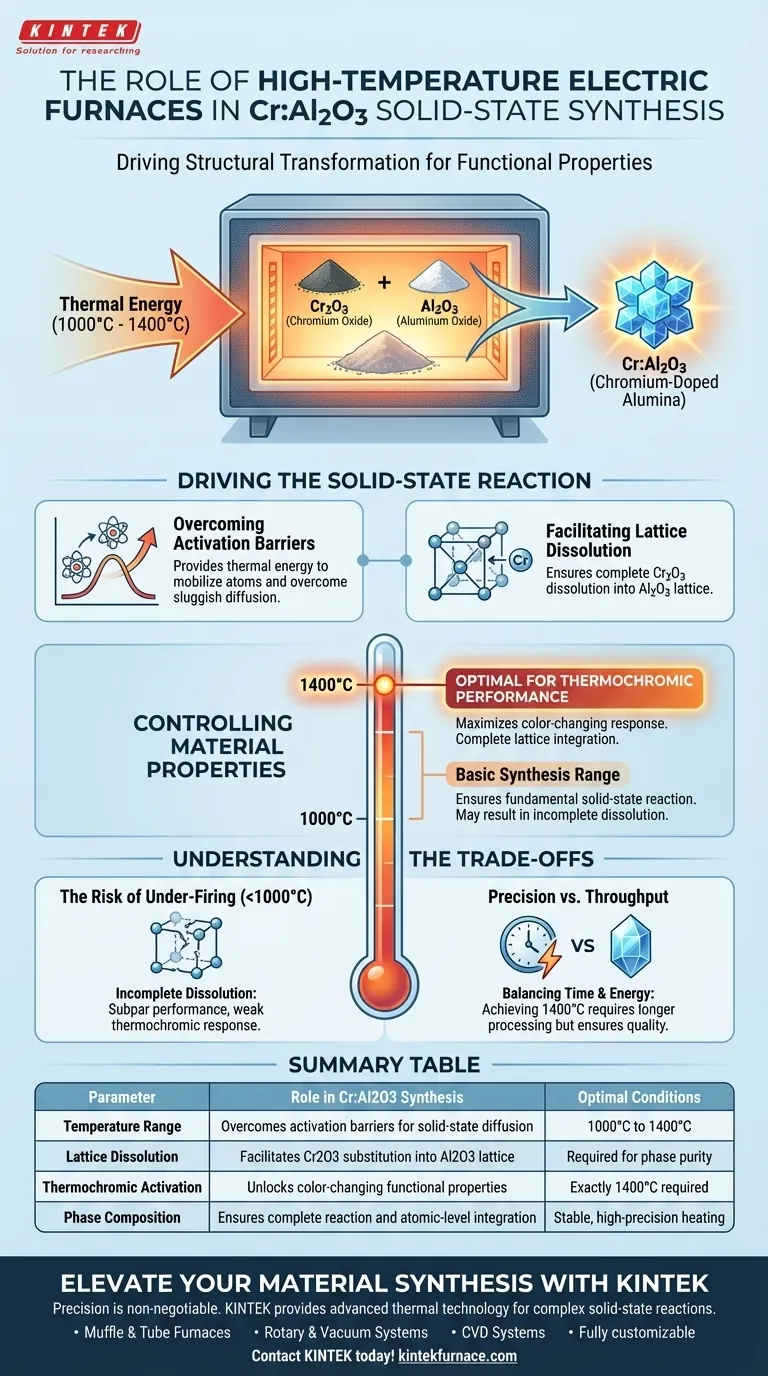
The high-temperature electric furnace acts as the primary driver of structural transformation during the synthesis of chromium-doped alumina. It provides the essential thermal energy, specifically between 1000°C and 1400°C, required to dissolve chromium oxide (Cr2O3) into the aluminum oxide (Al2O3) crystal lattice. This process is not merely about heating; it is about precisely controlling the phase composition to activate the material's specific functional properties.
Core Insight: The furnace does more than calcine the powder; it dictates the material's performance. Achieving a temperature of exactly 1400°C is critical to ensure complete lattice integration, which directly results in the optimal thermochromic (color-changing) response of the final powder.
Driving the Solid-State Reaction
The synthesis of chromium-doped alumina is a solid-state reaction, meaning the chemical change occurs without the materials melting into a liquid. The furnace facilitates this through specific mechanisms.
Overcoming Activation Barriers
Solid-state diffusion is inherently sluggish at room temperature. The furnace provides the thermal activation energy needed to mobilize atoms.
By maintaining temperatures above 1000°C, the furnace allows reactant particles to overcome their inertia. This enables the necessary atomic movement for the reaction to proceed at a practical rate.
Facilitating Lattice Dissolution
The core objective of this synthesis is the complete dissolution of Cr2O3 into the Al2O3 lattice.
The furnace creates an environment where chromium ions can effectively substitute for aluminum ions within the crystal structure. This atomic-level integration is what fundamentally changes the nature of the alumina powder.
Controlling Material Properties
The precision of the electric furnace directly correlates to the quality and functionality of the synthesized powder.
Determining Phase Composition
Temperature stability is vital for ensuring phase purity.
If the temperature fluctuates or fails to reach the necessary threshold, the dissolution process may be incomplete. This results in a mixture of unreacted oxides rather than a unified doped crystal structure.
Unlocking Thermochromic Performance
The primary functional goal of Cr:Al2O3 synthesis is often its thermochromic performance—its ability to change color with temperature.
The primary reference indicates that a treatment at 1400°C is specifically required to maximize this response. The furnace allows you to reach and hold this exact temperature to ensure the optical properties are fully developed.
Understanding the Trade-offs
While high temperatures are necessary, the process requires careful management to avoid common pitfalls.
The Risk of Under-Firing
Operating at the lower end of the range (near 1000°C) may initiate the reaction but fail to complete it.
Incomplete dissolution of the chromium leads to subpar performance. While the material may chemically resemble the target, it will lack the intense thermochromic response that defines high-quality chromium-doped alumina.
Precision vs. Throughput
Achieving the optimal 1400°C standard often requires longer processing times or more energy consumption compared to lower-temperature treatments.
You must balance the cost of energy and time against the strict requirement for material performance. Shortcutting the thermal profile in the furnace will invariably lead to a degradation in the material's color-changing capabilities.
Making the Right Choice for Your Goal
To achieve the best results with your high-temperature electric furnace, align your thermal profile with your specific performance requirements.
- If your primary focus is basic synthesis: Operate within the 1000°C to 1400°C range to ensure the fundamental solid-state reaction between Cr2O3 and Al2O3 occurs.
- If your primary focus is maximum thermochromic performance: You must configure the furnace to reach and maintain a stable 1400°C, as this specific temperature is required for optimal color-changing properties.
The furnace is not just a heat source; it is the precision tool that defines the atomic architecture of your final product.
Summary Table:
| Parameter | Role in Cr:Al2O3 Synthesis | Optimal Conditions |
|---|---|---|
| Temperature Range | Overcomes activation barriers for solid-state diffusion | 1000°C to 1400°C |
| Lattice Dissolution | Facilitates Cr2O3 substitution into Al2O3 lattice | Required for phase purity |
| Thermochromic Activation | Unlocks color-changing functional properties | Exactly 1400°C required |
| Phase Composition | Ensures complete reaction and atomic-level integration | Stable, high-precision heating |
Elevate Your Material Synthesis with KINTEK
Precision is non-negotiable when your material’s performance depends on exact lattice integration. KINTEK provides the advanced thermal technology required to master complex solid-state reactions.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of lab high-temp furnaces, including:
- Muffle & Tube Furnaces for precise batch processing.
- Rotary & Vacuum Systems for specialized atmosphere control.
- CVD Systems for advanced coating and synthesis.
All KINTEK systems are fully customizable to meet your unique research or production requirements. Ensure your chromium-doped alumina reaches its full thermochromic potential with our industry-leading heating solutions.
Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Eren Özmen, Mark D. Losego. Rapid, Direct Fabrication of Thermochromic Ceramic Composite Sensors via Flash Lamp Annealing. DOI: 10.1002/adem.202400323
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What features make muffle furnaces easy to operate? Discover Key Ease-of-Use Features
- What are the uses of muffle furnaces in calcination and sintering? Achieve Precise High-Temperature Material Transformations
- What are the typical specifications for lab box furnaces? Find Your Perfect Fit for Materials Processing
- What are some advancements in modern muffle furnace technology? Boost Precision and Efficiency in Your Lab
- Can a muffle furnace be used for metal heat treatments? Discover its capabilities and limitations for your lab.
- How long does heating take on a muffle furnace? From 25 Minutes to Hours Explained
- How do muffle furnaces contribute to energy efficiency? Discover Advanced Heat Management for Labs
- What are the environmental requirements for muffle furnace nanocrystallization of Fe-based alloys?



















