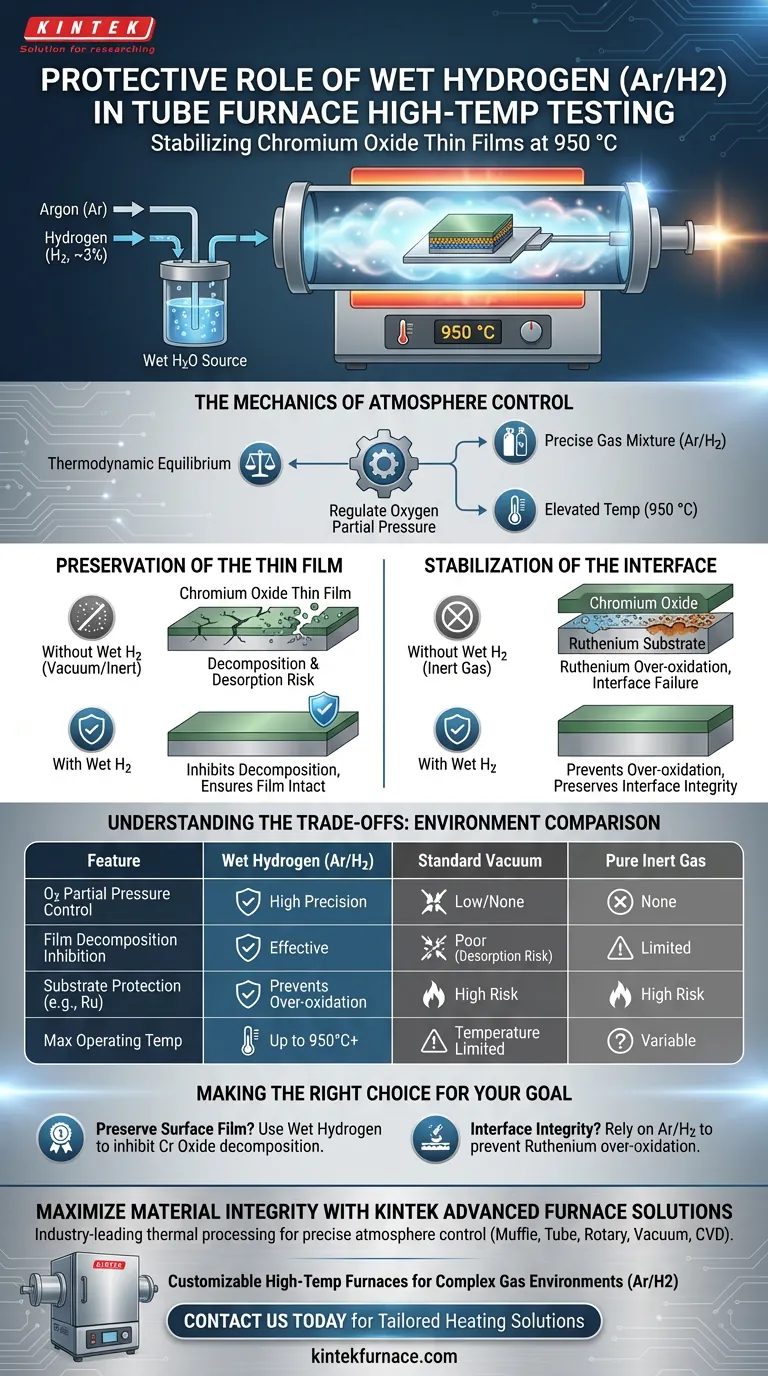
A flowing wet hydrogen (Ar/H2) environment serves as a critical stabilization medium during the high-temperature testing of chromium oxide thin films. By introducing a specific mixture, such as 3% Hydrogen in Argon, this atmosphere provides precise control over oxygen partial pressure at temperatures as high as 950 °C to prevent material degradation.
Core Takeaway Standard vacuum or inert gas environments are often insufficient for ultra-high temperature testing. A wet hydrogen atmosphere is chemically required to simultaneously inhibit the decomposition of the oxide film and prevent the over-oxidation of sensitive substrate layers.

The Mechanics of Atmosphere Control
Regulating Oxygen Partial Pressure
The primary function of a wet hydrogen environment in a tube furnace is the precise regulation of oxygen partial pressure.
At elevated temperatures (e.g., 950 °C), the chemical stability of thin films is highly dependent on the surrounding atmosphere.
By utilizing a mixture of Argon and Hydrogen (3%), the system establishes a thermodynamic equilibrium that maintains the specific oxygen levels required to stabilize the materials.
Preservation of the Thin Film
Inhibiting Decomposition
One of the most significant risks during high-temperature testing is the physical and chemical breakdown of the surface material.
Chromium oxide thin films are susceptible to decomposition and desorption under extreme heat.
The wet hydrogen environment effectively inhibits these processes, ensuring the film remains intact where vacuum or pure inert gases would fail.
Stabilization of the Interface
Protecting Underlying Ruthenium
In multi-layered structures, the stability of the interface between the film and the substrate is paramount.
Specifically, when a ruthenium layer underlies the chromium oxide, it is vulnerable to over-oxidation which can destroy the device structure.
The Ar/H2 environment prevents this over-oxidation, thereby preserving the thermal stability of the interface structure even at ultra-high temperatures.
Understanding the Trade-offs
Limitations of Alternative Environments
It is critical to understand why simpler environments are often rejected for this specific application.
Vacuum environments often lack the partial pressure control necessary to stop desorption.
Similarly, pure inert gases (like pure Argon) do not provide the chemical buffering required to prevent the underlying ruthenium from oxidizing. Therefore, while a wet hydrogen setup is more complex to implement, it is strictly necessary for accurate stability testing in this context.
Making the Right Choice for Your Goal
To ensure the validity of your high-temperature stability tests, you must select an environment that aligns with your material constraints.
- If your primary focus is preserving the surface film: Use wet hydrogen to specifically inhibit the decomposition and desorption of chromium oxide.
- If your primary focus is Interface Integrity: Rely on the Ar/H2 mixture to prevent the over-oxidation of underlying layers like ruthenium.
By controlling the oxygen partial pressure through a wet hydrogen flow, you ensure the survival of the entire material stack at 950 °C.
Summary Table:
| Feature | Wet Hydrogen (Ar/H2) | Standard Vacuum | Pure Inert Gas |
|---|---|---|---|
| Oxygen Partial Pressure Control | High Precision | Low/None | None |
| Film Decomposition Inhibition | Effective | Poor (Desorption Risk) | Limited |
| Substrate Protection (e.g., Ru) | Prevents Over-oxidation | High Risk | High Risk |
| Max Operating Temperature | Up to 950°C+ | Temperature Limited | Variable |
Maximize Material Integrity with KINTEK Advanced Furnace Solutions
Don't let material degradation compromise your research. KINTEK provides industry-leading thermal processing solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered for precise atmosphere control.
Our expert R&D team manufactures customizable high-temperature furnaces designed to handle complex gas environments like Ar/H2, ensuring your thin films and sensitive substrates remain stable at temperatures up to 950 °C and beyond.
Ready to elevate your lab's testing capabilities? Contact us today to discuss your unique project requirements and discover how our tailored heating solutions can deliver the precision your research demands.
Visual Guide
References
- Quintin Cumston, William E. Kaden. Wafer-scale development, characterization, and high temperature stabilization of epitaxial Cr2O3 films grown on Ru(0001). DOI: 10.1063/5.0201818
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the role of a dual-temperature zone tube furnace in MoS2 CVD growth? Mastering Precision 2D Synthesis
- What are the advantages of using a fixed-bed continuous flow tube reaction system? Unlock Precision CO2 Hydrogenation
- What is a tube furnace and where is it commonly used? Discover Precision Heating for Advanced Materials
- What is the principle of tube furnace? Master Precise High-Temp Environment Control
- What physical conditions does a laboratory Tube Furnace provide for SOEC? Precision Heat for Solid Oxide Characterization
- Why is annealing in a tube furnace essential for rGO-NiO-ZnO-400? Optimize Your Catalyst Synthesis
- What is the purpose of thermal insulation in a tube furnace? Ensure Uniform Heating and Energy Efficiency
- Why are vacuum-sealed quartz tubes essential for Bi-Sb-Te phase diagrams? Ensure Chemical Fidelity in Your Alloy Synthesis



















