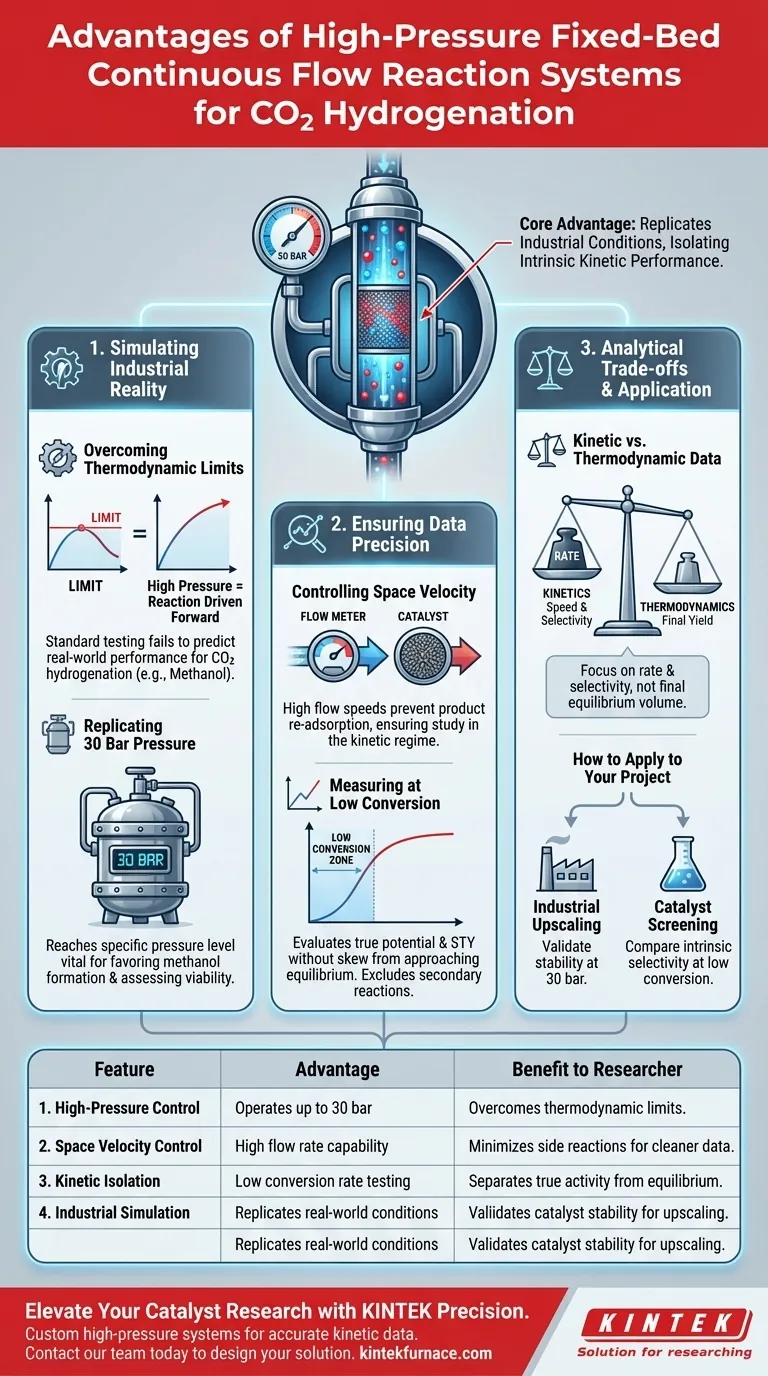
The primary advantage of using a fixed-bed continuous flow tube reaction system equipped with a high-pressure controller is its ability to replicate industrial operating conditions while isolating intrinsic catalyst performance. This setup enables the simulation of reaction pressures up to 30 bar, which is critical for overcoming thermodynamic equilibrium limitations inherent in processes like methanol synthesis. Additionally, it allows for precise control over space velocity, ensuring data accuracy by minimizing the interference of complex side reactions.
By maintaining high pressure and high space velocity, this system allows researchers to measure product selectivity and Space Time Yield (STY) at low conversion rates. This effectively separates the true kinetic activity of the catalyst from thermodynamic equilibrium states.

Simulating Industrial Reality
Overcoming Thermodynamic Limits
For CO2 hydrogenation, particularly methanol synthesis, the reaction is often limited by thermodynamic equilibrium. Standard low-pressure testing cannot accurately predict how a catalyst will perform in a real-world scenario.
Replicating 30 Bar Pressure
A high-pressure controller allows the system to reach and maintain pressures of 30 bar. This specific pressure level is vital for driving the reaction forward, favoring the formation of methanol and allowing for a realistic assessment of industrial viability.
Ensuring Data Precision
Controlling Space Velocity
The system allows for high space velocity control. This ensures that reactants flow over the catalyst bed at a speed that prevents the re-adsorption of products, which is essential for studying the reaction in its kinetic regime.
Measuring at Low Conversion
To understand a catalyst's true potential, it is best evaluated at low conversion rates. This system facilitates these specific conditions, allowing for the accurate calculation of Space Time Yield (STY) without the data becoming skewed by the approach to equilibrium.
Excluding Complex Side Reactions
By operating at high space velocities and controlled pressures, the system effectively excludes the influence of secondary reactions. This ensures that the measured selectivity is a result of the primary catalytic mechanism, not downstream chemical noise.
Understanding the Analytical Trade-offs
The "Low Conversion" Constraint
While evaluating at low conversion rates is excellent for determining reaction kinetics, it does not display the maximum total yield possible in a single pass.
Kinetic vs. Thermodynamic Data
This setup is optimized to provide data on the rate and selectivity (kinetics) rather than the final equilibrium composition. Researchers must understand that this data represents the catalyst's speed and specificity, not necessarily the final product volume of a full-scale reactor operating at maximum conversion.
How to Apply This to Your Project
- If your primary focus is Industrial Upscaling: Utilize the 30 bar pressure capability to validate that your catalyst remains stable and active under commercially relevant conditions.
- If your primary focus is Catalyst Screening: Use high space velocity to maintain low conversion rates, allowing you to compare the intrinsic selectivity and STY of different materials without equilibrium interference.
This system is the definitive tool for distinguishing between a catalyst's theoretical limits and its actual kinetic speed.
Summary Table:
| Feature | Advantage | Benefit to Researcher |
|---|---|---|
| High-Pressure Control | Operates up to 30 bar | Overcomes thermodynamic limits for methanol synthesis. |
| Space Velocity Control | High flow rate capability | Minimizes side reactions and re-adsorption for cleaner data. |
| Kinetic Isolation | Low conversion rate testing | Separates true catalyst activity from equilibrium states. |
| Industrial Simulation | Replicates real-world conditions | Validates catalyst stability for commercial upscaling. |
Elevate Your Catalyst Research with KINTEK Precision
Are you looking to bridge the gap between lab-scale discovery and industrial reality? KINTEK provides state-of-the-art fixed-bed continuous flow systems and high-pressure tube reactors designed to handle the rigorous demands of CO2 hydrogenation and methanol synthesis.
Backed by expert R&D and manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique pressure and temperature requirements. Our high-pressure controllers and precision flow systems ensure you get the accurate kinetic data needed for successful catalyst screening and upscaling.
Ready to optimize your reaction performance? Contact our engineering team today to design a custom high-temperature system tailored to your research goals.
Visual Guide
References
- C. Romero, R.M. Navarro. Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites. DOI: 10.3390/catal15010055
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- What roles does a tube furnace play during the high-temperature calcination? Engineering Cobalt-Free Cathodes
- What is a tube furnace and what are its primary uses? Essential for Controlled High-Temperature Processes
- How do temperature control and uniformity differ between vertical and horizontal tube furnaces? Optimize Your Lab's Heat Processing
- What are the key benefits of using a tube furnace for material processing? Achieve Precise Heat Control for Superior Results
- How does a tube furnace facilitate gas-phase hydrogenation for Zircaloy-4? Achieve Precise Hydride Precipitation
- Why is the space-saving design of a tube furnace advantageous? Unlock Efficiency in Your Lab
- Why is a Quartz Tube Furnace with Gas Flow Control Required for Iodine Doping? Precision Single-Atom Catalyst Synthesis
- Why is a horizontal tube furnace utilized for BPEA growth? Mastering Physical Vapor Transport for Single Crystals



















