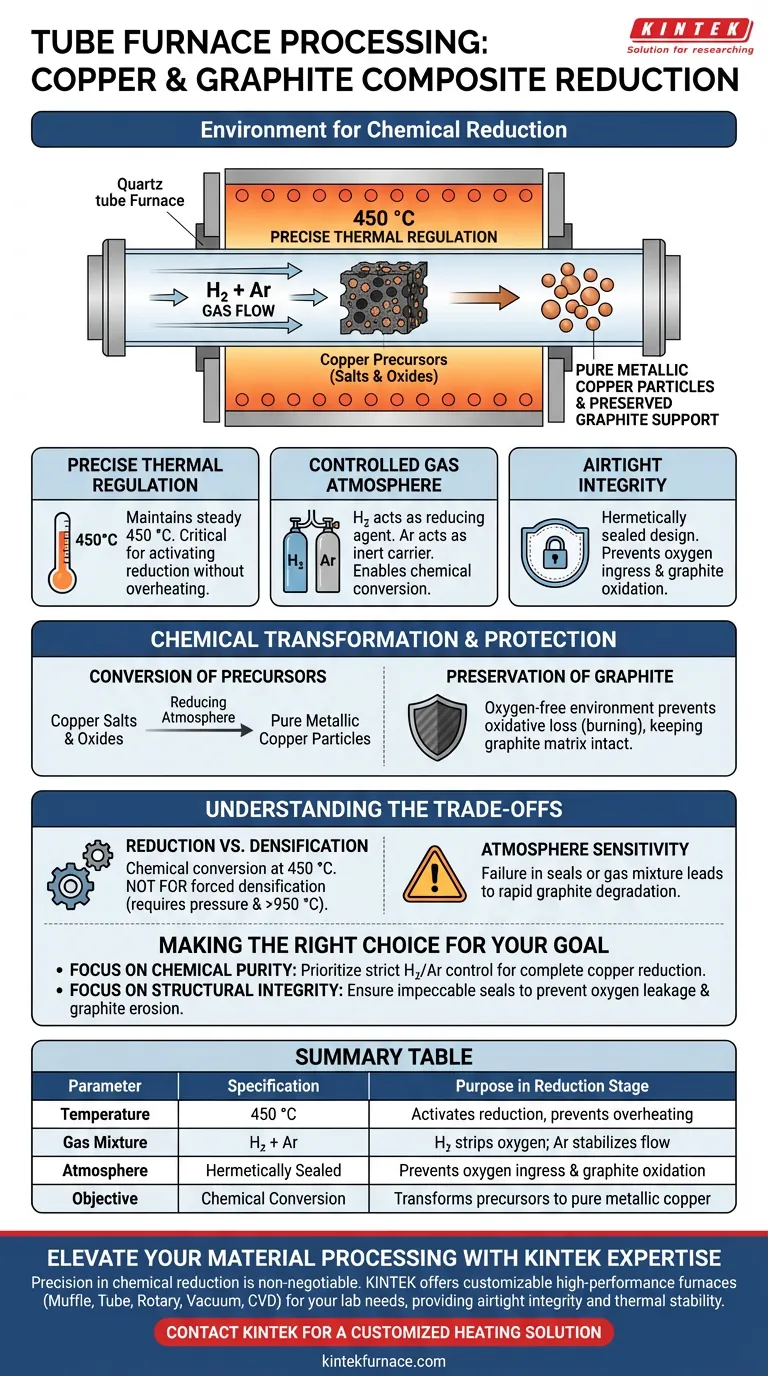
A tube furnace provides a hermetically sealed, precisely heated environment specifically designed for chemical reduction. For copper and graphite composites, this involves maintaining a constant temperature of 450 °C while circulating a specific mixture of reducing gases, such as hydrogen and argon, to facilitate chemical transformation.
The core function of this environment is to convert copper precursors into metallic copper while simultaneously protecting the graphite matrix from oxidation through a strictly controlled, airtight atmosphere.

The Mechanics of the Reduction Environment
Precise Thermal Regulation
The furnace maintains a steady temperature of 450 °C.
This specific thermal plateau is critical for activating the reduction reaction without overheating the composite components.
Controlled Gas Atmosphere
The environment relies on a flow of reducing gases, typically a mixture of hydrogen and argon.
Hydrogen acts as the active reducing agent to strip oxygen from the copper compounds, while argon serves as an inert carrier gas to stabilize the atmosphere.
Airtight Integrity
The tube furnace utilizes a specialized sealing design to ensure the chamber remains airtight.
This prevents the ingress of ambient oxygen, which is essential for maintaining the purity of the internal atmosphere.
Chemical Transformation and Protection
Conversion of Precursors
The primary goal of this stage is the chemical conversion of copper salts and oxides.
Under these conditions, the reducing atmosphere facilitates the transformation of these impregnated powders into pure metallic copper particles.
Preservation of Graphite
Graphite is highly susceptible to oxidative loss (burning) at high temperatures if exposed to air.
The oxygen-free environment provided by the tube furnace ensures the graphite support remains intact throughout the heating process.
Understanding the Trade-offs
Reduction vs. Densification
It is critical not to confuse the reduction stage with the final sintering stage.
While the tube furnace is excellent for chemical conversion at 450 °C, it does not provide the mechanical pressure or ultra-high temperatures (e.g., 950 °C) required for the forced densification of the copper-graphite composite.
Atmosphere Sensitivity
The process is highly sensitive to the integrity of the gas supply and seals.
Any failure in the airtight construction or the gas mixture ratios can lead to rapid oxidative degradation of the graphite, rendering the composite unusable.
Making the Right Choice for Your Goal
To ensure the successful processing of copper and graphite composites, apply the following parameters:
- If your primary focus is chemical purity: Prioritize strict control over the hydrogen/argon gas mixture to ensure complete reduction of copper oxides.
- If your primary focus is structural integrity: Ensure the furnace seals are impeccably maintained to prevent oxygen leakage and the subsequent erosion of the graphite matrix.
Success in this stage depends on balancing precise thermal control with a flawless reducing atmosphere.
Summary Table:
| Parameter | Specification | Purpose in Reduction Stage |
|---|---|---|
| Temperature | 450 °C | Activates reduction without composite overheating |
| Gas Mixture | Hydrogen + Argon | Hydrogen strips oxygen; Argon stabilizes the flow |
| Atmosphere | Hermetically Sealed | Prevents oxygen ingress and graphite oxidation |
| Objective | Chemical Conversion | Transforms copper precursors into pure metallic copper |
Elevate Your Material Processing with KINTEK Expertise
Precision in chemical reduction is non-negotiable for high-performance composites. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique lab requirements. Whether you are processing copper-graphite composites or developing new advanced materials, our furnaces provide the airtight integrity and thermal stability you need.
Contact KINTEK today for a customized heating solution
Visual Guide
References
- Hiroshi Itahara, Yasuhiro Takatani. Facile synthesis of electrocatalytically active Cu/graphite using the negative electrode of spent Li-ion batteries. DOI: 10.1039/d3gc04472f
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- Why is the control of heating and cooling rates in a tube furnace critical for the thermal reduction of lithium niobate?
- What is the primary function of a high-temperature tube furnace in NaF–Na3AlF6 molten salt experiments? Learn more!
- What is the role of a tubular furnace in the conversion of coffee ground powder into biochar? Master Precise Pyrolysis
- What is a tubular furnace and what are its primary uses? Essential for High-Temperature Precision and Uniformity
- How does treatment in a high-temperature tube furnace affect TiOx@C precursors? Engineering Oxygen Vacancies
- What is the Purpose of Carbon Coating Quartz Tubes? Enhance Crystal Growth via Bridgman Method
- Why is a controlled nitrogen atmosphere necessary within a tube furnace during the annealing of Antimony-doped thin films?
- How does a dual-zone tube furnace control CoTeO4 crystal growth? Precision CVT Thermal Gradient Methods



















