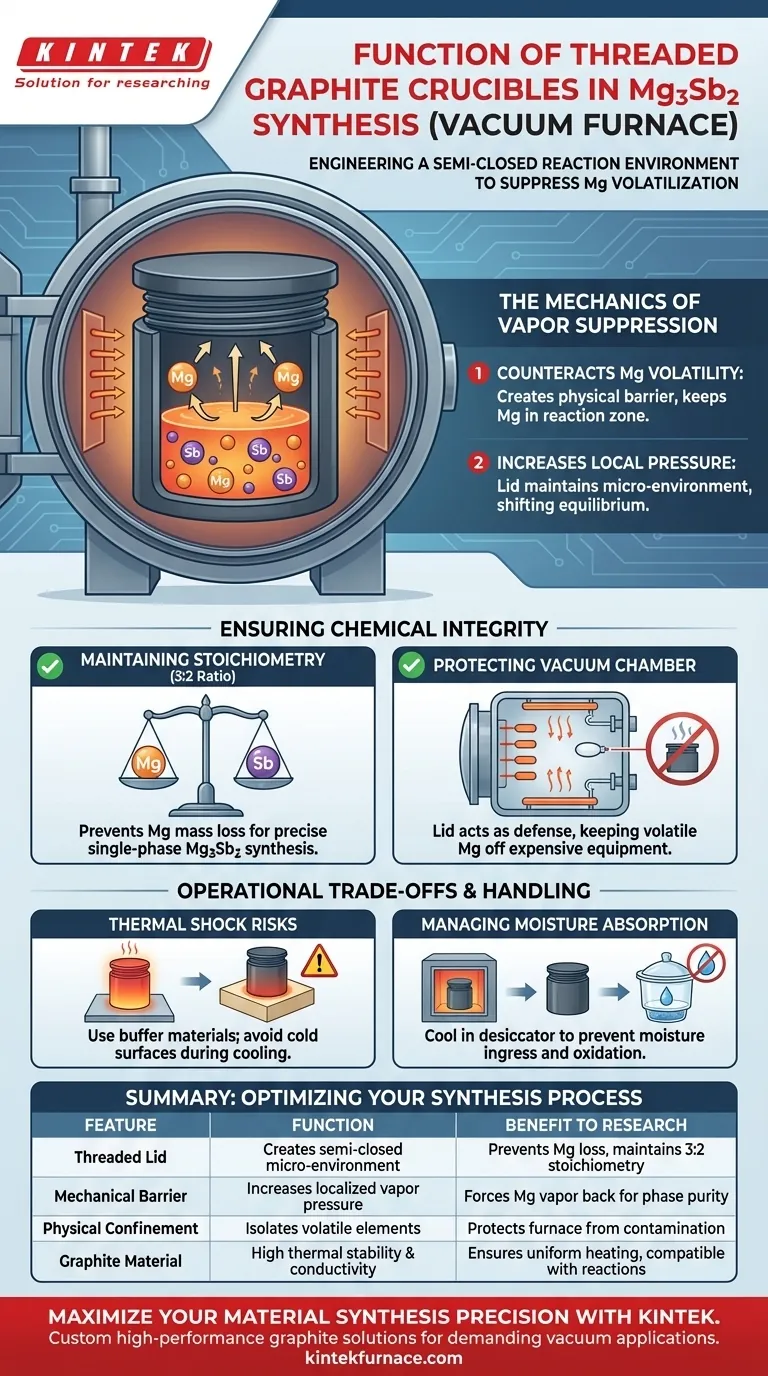
The specific function of a graphite crucible with a threaded lid is to engineer a contained, semi-closed reaction environment within your vacuum furnace. This mechanical barrier is designed primarily to suppress the rapid volatilization of Magnesium (Mg) at high temperatures, preventing material loss and ensuring the successful formation of the Mg3Sb2 compound.
By constructing a confined reaction space, the threaded lid counteracts the high vapor pressure of Magnesium. This preserves the precise stoichiometric ratio necessary for single-phase synthesis and prevents volatile elements from contaminating the vacuum chamber.

The Mechanics of Vapor Suppression
Counteracting Magnesium Volatility
Magnesium is highly volatile and prone to evaporation at the temperatures required to melt and react with Antimony (Sb). Without physical confinement, Mg atoms would escape the melt pool before the reaction is complete. The threaded lid creates a physical barrier that keeps Mg vapor within the immediate reaction zone.
Increasing Local Pressure
While the furnace itself operates under a vacuum, the interior of the crucible maintains a localized higher pressure due to the lid. This "micro-environment" shifts the equilibrium, forcing the Magnesium vapor to re-interact with the melt rather than dispersing into the vacuum chamber.
Ensuring Chemical Integrity
Maintaining Stoichiometry
Synthesizing Mg3Sb2 requires a strict atomic ratio of 3 parts Magnesium to 2 parts Antimony. If Magnesium escapes due to volatilization, the final compound will be Mg-deficient, resulting in a failed or impure single-phase compound. The threaded lid locks the mass inside, ensuring the final product matches your calculated input weights.
Protecting the Vacuum Chamber
Volatile components that escape a crucible do not simply disappear; they condense on the cooler parts of your furnace. This can coat heating elements and sensors, leading to cross-contamination or equipment failure. The lid serves as the first line of defense, keeping the volatile mess inside the crucible and off your expensive equipment.
Operational Trade-offs and Handling
Thermal Shock Risks
While graphite is excellent for high-temperature applications, it requires careful handling during the cooling phase. As noted in standard protocols, hot crucibles should be placed on buffer refractory materials rather than cold surfaces to prevent cracking from thermal shock.
Managing Moisture Absorption
Graphite is naturally porous and prone to absorbing atmospheric moisture. After the heating cycle, the crucible should be transferred to a desiccator for cooling. This prevents moisture ingress, which could cause oxidation or structural failure during subsequent heating cycles.
Optimizing Your Synthesis Process
To ensure the highest quality Mg3Sb2 synthesis, apply the following guidelines based on your immediate goals:
- If your primary focus is Phase Purity: Rely on the threaded lid to maintain the exact 3:2 stoichiometric ratio by preventing Mg mass loss.
- If your primary focus is Equipment Maintenance: Utilize the lid to prevent volatile Mg vapors from coating and degrading your vacuum furnace internals.
- If your primary focus is Process Consistency: Implement a strict cooling protocol using a desiccator to prevent moisture variables from affecting weight measurements.
The threaded lid is not merely a cover; it is a critical process control tool that enables the precise synthesis of volatile compounds.
Summary Table:
| Feature | Function in Mg3Sb2 Synthesis | Benefit to Research |
|---|---|---|
| Threaded Lid | Creates a semi-closed micro-environment | Prevents Mg loss & maintains 3:2 stoichiometry |
| Mechanical Barrier | Increases localized vapor pressure | Forces Mg vapor back into the melt for phase purity |
| Physical Confinement | Isolates volatile elements | Protects furnace heating elements & sensors from contamination |
| Graphite Material | High thermal stability & conductivity | Ensures uniform heating and compatibility with high-temp reactions |
Maximize Your Material Synthesis Precision with KINTEK
Don't let volatile material loss compromise your research integrity. KINTEK provides high-performance, customizable graphite solutions specifically engineered for demanding vacuum furnace applications. Backed by expert R&D and manufacturing, we offer high-purity Graphite, Muffle, Tube, Rotary, Vacuum, and CVD systems—all designed to maintain precise atmospheric control and protect your equipment.
Ready to optimize your high-temperature synthesis? Contact KINTEK today to discuss your custom crucible and furnace requirements with our technical experts.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- Why is Beryllium Oxide (BeO) used as a viscometer crucible? Superior Stability for High-Temperature Alloy Research
- What is the primary function of an industrial vacuum drying oven in Si-RuO2 catalyst preparation? Achieve Uniformity.
- How does a Rapid Thermal Annealing (RTA) system differ from a standard hotplate? Optimize Perovskite Crystallization
- What is the function of PTFE sealing rings in plastic pyrolysis? Ensure Safe, Anaerobic Material Decomposition
- What roles do high-purity graphite molds play in the SPS of copper sulfide? Enhance Your Thermoelectric Material Quality
- Why is a stainless steel crucible selected for melting AM60 magnesium alloy? Ensure Alloy Purity and Safety
- What are the structural functions of the dual-chamber quartz glass container? Optimize Magnesium Alloy Vapor Analysis
- What is the function of an enhanced hydrothermal reactor with magnetic stirring? Optimize MoS2/C Synthesis Yield














