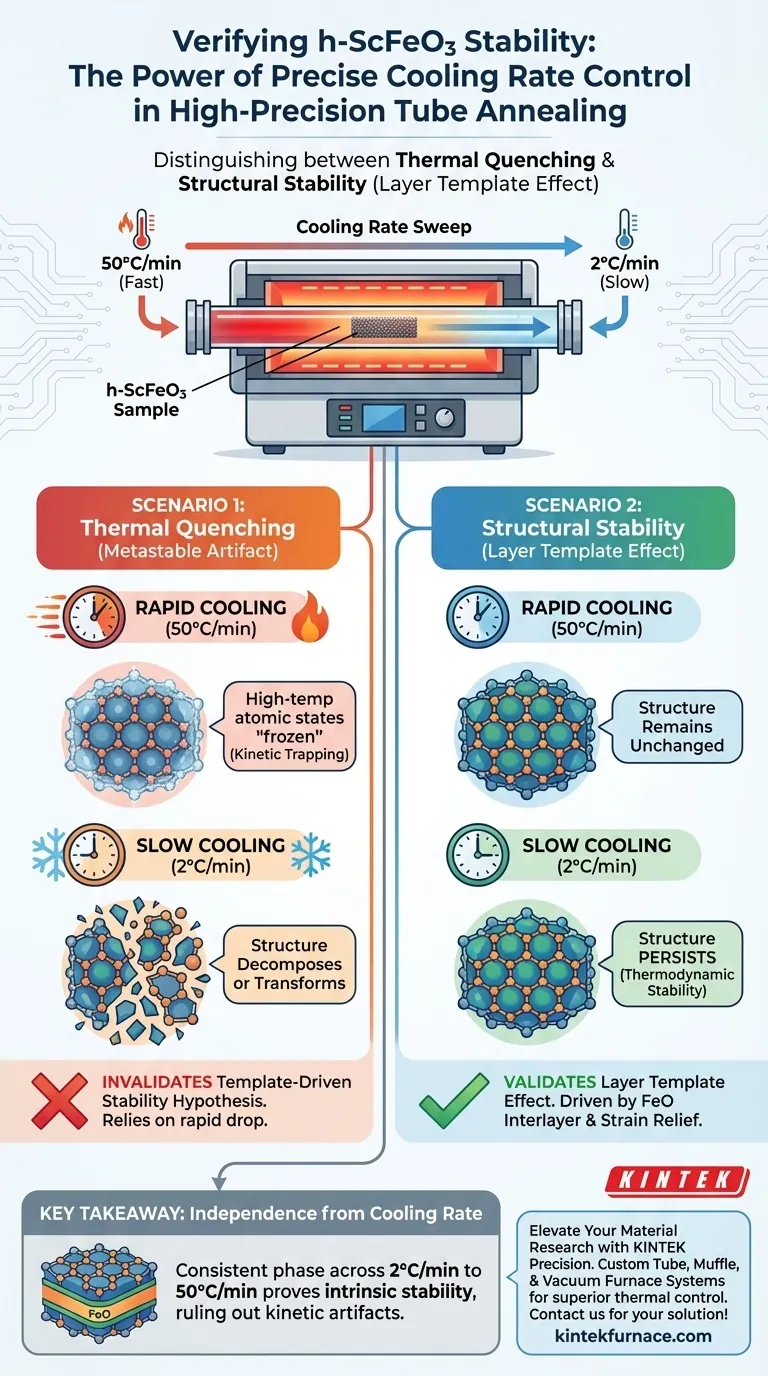
Precise control of cooling rates is the definitive method for distinguishing between thermally quenched artifacts and structurally stabilized phases. In the verification of h-ScFeO3, varying the cooling rate in a high-precision tube annealing furnace allows researchers to determine whether the material's structure is a result of rapid temperature drops or genuine structural engineering.
By sweeping cooling rates from 2°C/min to 50°C/min, researchers can prove that h-ScFeO3 stability is driven by the layer template effect of the FeO interlayer rather than being a metastable product of thermal quenching.

The Mechanism of Phase Verification
Eliminating the Quenching Variable
Thermal quenching occurs when a material is cooled so rapidly that its high-temperature atomic arrangement is "frozen" in place before it can rearrange into a stable low-temperature form.
By utilizing a wide range of cooling rates, specifically between 2°C/min and 50°C/min, the furnace tests whether the material relies on this rapid thermal drop to maintain its structure.
If the hexagonal phase relies on quenching, it would likely decompose or transform when cooled slowly.
Proving Structural Stability
If the h-ScFeO3 phase remains unchanged regardless of the cooling speed, it indicates the phase is not merely a kinetic artifact.
Persistence during slow cooling (2°C/min) proves that the material is not thermodynamically desperate to revert to a different phase as the temperature lowers.
This independence from thermal history highlights that the stability is intrinsic to the local environment of the material.
Validating the Template Effect
The primary goal of this test is to confirm the role of the FeO interlayer.
When the phase persists across all cooling rates, it provides conclusive evidence that the formation is driven by the layer template effect.
It confirms that strain relief mechanisms provided by the interlayer, rather than thermal manipulation, are responsible for holding the h-ScFeO3 structure together.
Methodological Considerations and Trade-offs
The Necessity of Dynamic Range
Testing a single cooling rate is insufficient for conclusive validation.
A "slow" rate alone might not be slow enough to trigger decomposition in highly metastable materials, while a "fast" rate alone proves nothing about thermodynamic stability.
You must employ a wide dynamic range (comparing 2°C/min against 50°C/min) to fully bracket the material's behavior.
Interpreting Phase Decomposition
It is critical to understand the implications of a "failed" test.
If the h-ScFeO3 phase were to alter or disappear during the slow cooling cycle, the hypothesis of template-driven stability would be invalidated.
This would force a re-evaluation of the FeO interlayer's effectiveness, suggesting it is not providing sufficient strain relief to stabilize the hexagonal phase without kinetic trapping.
Interpreting Stability Data for Material Design
Use the cooling rate data to validate your synthesis strategy and the effectiveness of your substrate engineering.
- If your primary focus is verifying the FeO interlayer: Look for phase consistency at 2°C/min to prove the template effect is the dominant stabilizing force.
- If your primary focus is ruling out metastable artifacts: Compare the crystal structure of the 50°C/min sample against the 2°C/min sample; identical structures confirm the absence of thermal quenching.
Ultimately, independence from cooling rate is the hallmark of a phase stabilized by structural engineering rather than thermal manipulation.
Summary Table:
| Feature | Rapid Cooling (50°C/min) | Slow Cooling (2°C/min) |
|---|---|---|
| Primary Function | Tests for thermal quenching artifacts | Verifies thermodynamic stability |
| Effect on h-ScFeO3 | "Freezes" high-temp atomic states | Allows for potential phase decomposition |
| Verification Goal | Rule out kinetic trapping | Confirm FeO interlayer template effect |
| Stability Indicator | Structural persistence is expected | Structural persistence proves intrinsic stability |
Elevate Your Material Research with KINTEK Precision
Are you struggling to distinguish between metastable artifacts and genuine structural stability? KINTEK’s high-precision Tube, Muffle, and Vacuum furnace systems provide the industry-leading thermal control and dynamic cooling ranges (from 2°C/min to 50°C/min) essential for validating advanced materials like h-ScFeO3.
Backed by expert R&D and specialized manufacturing, our systems are fully customizable to meet the unique needs of your lab. Whether you require CVD systems or rotary furnaces, KINTEK offers the stability and uniformity necessary for world-class results.
Ready to achieve superior heat treatment for your lab? Contact us today to discuss your custom furnace solution!
Visual Guide
References
- Marshall B. Frye, Lauren M. Garten. Interlayer‐Mediated Stabilization of Metastable <i>P</i>6<sub>3</sub><i>cm</i> ScFeO<sub>3</sub> on Al<sub>2</sub>O<sub>3</sub>. DOI: 10.1002/admi.202500114
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- What optional accessories are available for three-zone split tube furnaces? Enhance Control and Efficiency for Your Lab
- What role does an industrial-grade tube furnace play in Fe-P-NC catalyst pyrolysis? Precision Heating for Fe-P-NC Synthesis
- What role does a high-temperature tube furnace play in biomass carbonization? Unlock Superior Biochar Structures
- Why is control of heating rate and gas flow in a lab tube furnace critical for EM wave absorption materials?
- What is the function of sealed quartz ampoules in Se80In5Te6Sb9 synthesis? Ensure Purity and Precision
- What are the primary functions of a high-performance tube furnace in the two-stage synthesis of Ln-MoP@C catalysts?
- What is the role of a three-zone tube furnace in HPHT nanodiamond pretreatment? Unlock Precise Surface Activation



















